Hydration of Alkenes
HYDRATION OF ALKENES
Alcohols can be produced from alkenes via several mechanisms. One of the most common method of preparation of alcohol is acid-catalyzed hydration of alkenes. This reaction follows the Markovnikov’s rule. This rule is followed by addition reactions whose mechanism involves having an H atom added to the less substituted carbon. In the case of acid-catalyzed hydration, following the Markovnikov’s rule also leads to production of the more stable carbocation.
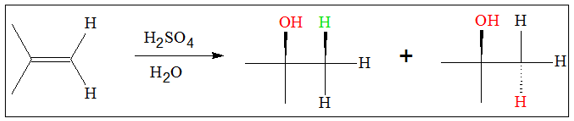
The mechanism for the reaction proceeds through the following mechanism:
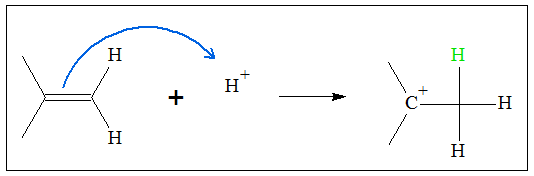
- 1. An acid catalyst (proton) is added to the alkene. Because of the nucleophilic nature of the double bond of the alkene, it can donate an electron pair to the electrophilic proton. In this way, the pi electrons are removed and the H atom forms a pi bond with one of the carbon atoms in the C=C group. Since the addition follows Markovnikov’s rule, the proton is added to the less substituted carbon. The other carbon atom of C=C will bear a positive charge at the end of the addition of the proton.
- 2. Since there is a localized positive charge on a carbon atom, water molecules, bearing lone pairs, can donate one of its lone pairs to the positively charged carbon. The end-product of the process is an intermediate where the oxygen atom has a positive charge.
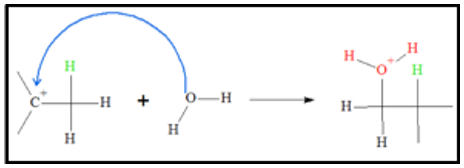
- 3. Since oxygen atoms are electronegative, they don’t like to bear positive charges. Because of this, the tendency for the oxygen atom is to give up one of its proton. When the proton leaves, an alcohol is produced and the acid catalyst is regenerated.
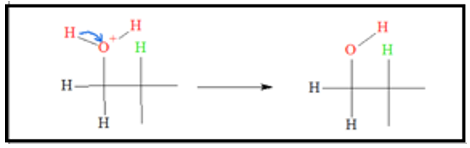
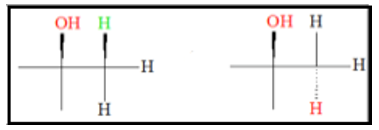
The product of acid-catalyzed hydration is not stereospecific. This means addition of the OH group can be either from the top or bottom of the original double bond. Because of this, a mixture of stereoproducts is expected.