Epoxidation of Alkenes
EPOXIDATION OF ALKENES
Epoxides are cyclic ethers with a three membered ring composed of two carbon atoms and one oxygen atom. The structure approximates an equilateral triangle. Because of its configuration, the compound is very reactive because of the high strain present in the bonds of the compounds. Unlike other ethers which can be used as an organic solvent, epoxides or cyclic ethers are not normally used as solvents because of its high reactivities. The common structure for an epoxide is shown below.

Epoxides can be prepared by a number of different reactions. These include heterogenous catalyzed oxidation of alkenes, olefin oxidation using peroxides and metal catalysts, homogenously catalyzed asymmetric epoxidations, nucleophilic epoxidation, biosynthesis, and olefin peroxidation using peroxycarboxylic acid. In this article, we will focus on epoxidation of alkenes using peroxycarboxylic acid.
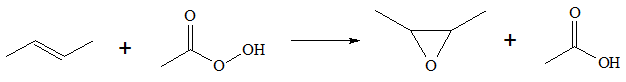
Peroxy acids are acids, which contains and –OOH group. Compared to a normal carboxylic acid, an extra oxygen is present between the carbonyl carbon and the –OH group. Peroxy acids are about 1000x weaker than normal carboxylic acid. This is due to the fact that peroxy acid cannot undergo resonance stabilization of its anion. This compound is commonly used in converting alkenes to epoxides. Theya re also used in oxidizing amines and thioetheres to amine oxides and sulfoxides. It is used as the main reagent in the Baeyer-Villiger Oxidaion of alkenes.
In the epoxidation of alkenes, one of the oxygen atom is transferred from the peroxyacid to the carbon-carbon double bond forming the oxirane ring. One common peroxyacid used is meta-chloroperoxybenzoic acid (m-CPBA). This reactions is called Prilezhaev epoxidation. It is named after Nikolaus Prileschajew who reported the reaction in 1909. M-CPBA is a good reagent to use because of its stability and good solubility in organic solvents. Below is an example of epoxidation reaction using peroxybenzoic acid.
The reaction proceeds via the “Butterfly mechanism.” In this mechanism, the peroxide acts as an electrophile, while the alkene acts as the nucleophile. The reaction is said to be a concerted reaction, which means that bond breaking and bond making occurs in a single step. The mechanism for the oxidation process is shown below.
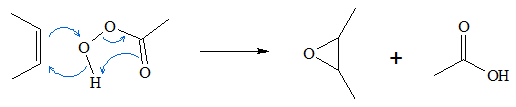
This mechanism works if the reactants arrange themselves properly in the reaction system. The hydroxyl oxygen should be properly oriented at top of the olefinic carbons to enable simultaneous bond making forming the oxirane ring. As the C-O bonds are formed, the pi bonds between the C=C disappears. At the same time, the hydroxyl hydrogen form new bond with the carbonyl oxygen. Rearrangements lead to formation of the carboxylic acid and the epoxide.
