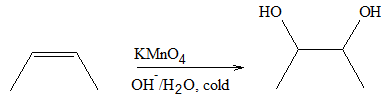Dihydroxylation
DIHYDROXYLATION
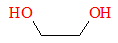
Dihydroxylation is the process by which an alkene is converted into a vicinal diol. Vicinal diols are a type of organic compounds containing hydroxyl groups in adjacent carbon atoms. These compounds are called glycols. An example of which is 1.2-ethanediol, or ethylene glycol. Diols are important as there are some polymers that uses diols as their monomers. Below is the structure of ethylene glycol.
The common method of preparation of dihydroxylated compound is through oxidation of alkenes. Unlike most oxidation process, a transition metal is usually used as catalyst for this reaction. Osmium tetroxide is commonly used to synthesize 1,2-diols. The general reaction is shown below.

The general mechanism for the syn dihydroxylation of alkenes using osmium tetroxide involves formation of a cyclic ester. Below is an outline of the process of dihydroxylation.
- 1. Osmium tetroxide is added to the alkene group forming osmate ester, which is a five-sided cyclic intermediate.
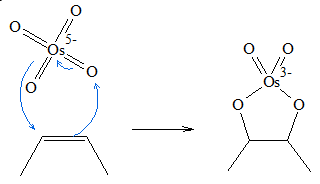
- 2. Water then attacks the osmium atom of the intermediate. During the process of the attack, as soon as the oxygen atom of the water molecule attaches itself to the Osmium atom, one of the hydrogen atoms of the water molecule is transferred to the oxygen atom attached to the original olefinic carbon.
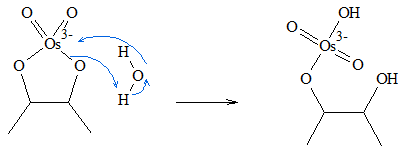
- 3. Another water molecule attacks the other side of the ester. When the reaction is complete, the syn-diol product is formed.
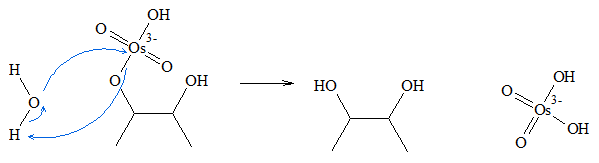
Dihydroxylation is stereospecific, which means the addition of the hydroxyl groups occur in specific orientation. Since a cyclic intermediate is formed, the two hydroxyl groups are added in syn positions (same side).
Syn diols can also be produced using potassium permanganate. However, since the potassium permanganate is a strong oxidant, sometimes it leads to cleavage of the diol after further oxidation.