Oxymercuration of Alkenes
OXYMERCURATION OF ALKENES
Another method of producing alcohol is oxymercuration. This process is usually done in small-scale hydration. What facilitates the addition process are salts like mercuric acetate. The general reaction involve in oxymercuration is shown below.
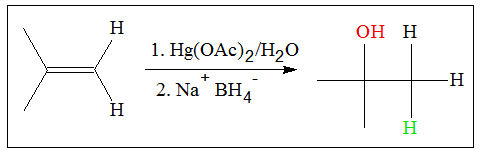
The process of alcohol production through oxymercuration is a two-step process. The mechanism of addition of water follows the following mechanism:
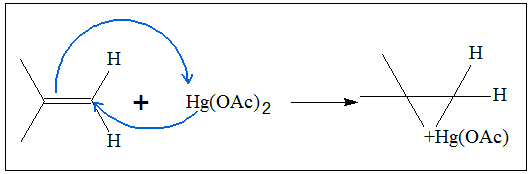
- 1. The first step in oxymercuration is the formation of a cyclic mercurinium ion. The mercury atom of the added salt form bonds with each of the carbon in the C=C double bond. In this case, a cyclic three-membered ring is produced. Below is the mechanism of producing the mercurinium ion.
- 2. Since there is a charge in the mercury atom, and with the steric strain brought about by the cyclic chain, a water molecule can readily attack the carbon atoms. It is noted that the attack of the water molecule occurs at the more substituted carbon.
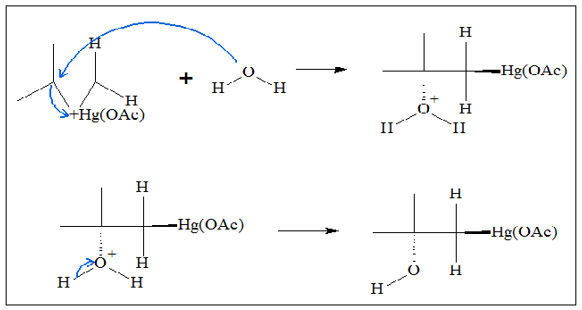
Because of the groups around the more substituted carbon, the tendency for the molecule is to produce a C-Hg bond that is longer compared to the C-Hg bond in the less-substituted carbon. Because of the longer C-Hg bond in the more substituted carbon, the bond in that part of the chain is weaker, which means it is easier for a water molecule to attack the carbon atom, eventually releasing the C-Hg bond. In the process, what is produced is a mercury-containing alcohol.
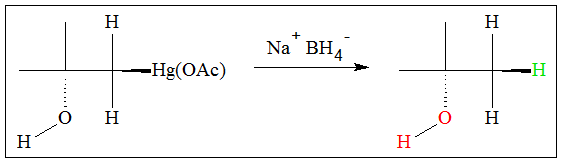
The mercury-containing alcohol can be further processed to remove the mercury. Using sodium borohydride (NaBH4). The molecule is reduced and the mercury group is removed and is replaced by a hydrogen atom.