Carbene Reaction
CARBENE REACTION
Carbenes are neutral divalent carbon compounds. They contain a neutral carbon atom with a valence of two and two unshared valence electrons. Sometimes the term carbine is used to refer to the specific compound called methylene. The structure for methylene is shown below.
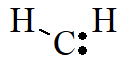
Methylene is the parent hydride from which the other carbene compounds are produced. Most of the carbene molecules are very short lived. This is because these compounds are highly unstable. As soon as they are formed, they react immediately with other molecules.
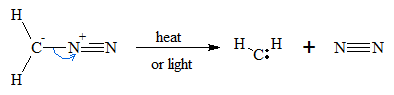
Methylene can be prepared by the decomposition of diazomethane (CH2N2). The decomposition is accomplished by thermolysis of diazomethane (heating) or by irradiation (photolysis). The general reaction in the decomposition of diazomethane is shown below.
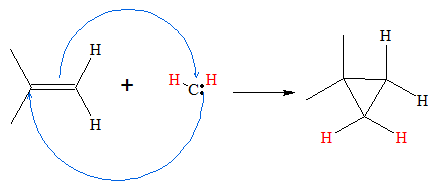
Carbenes are very reactive that it readily can react with alkenes. When reacted with alkenes, the carbene molecule adds to the double bond forming a cyclopropane molecule. This is achieved by having the olefinic carbons donating a pair of electron to the carbon atom of carbene. At the same time, the carbene molecule donates its lone pair of electron to one of the olefinic carbons forming a three-sided cyclic chain. Below is the mechanism of cyclopropane synthesis via addition of carbene to an alkene.
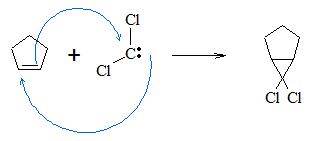
There are also other carbene molecules, which do not contain hydrogen atoms. Dihalocarbenes are type of carbene compounds containing two halo groups. They are synthesized by the elimination of hydrogen halide from a haloform. The reaction forming dihalocarbene is similar to that of the β-elimination reaction, which forms alkenes from alkyl halides. Below is the general reaction for this process.
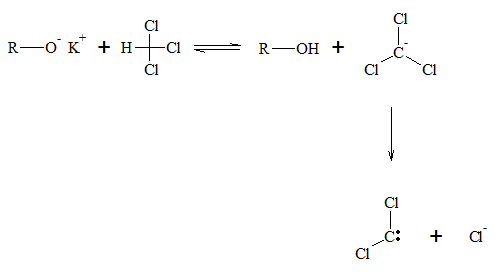
Dihalocarbenes can also be added to alkenes with the same mechanism as methylene. The addition of the carbene is stereospecific, which means trans alkene, will still have their R groups in trans position from each other after formation of the cyclopropane product, while cis alkenes will still have their R groups in cis position after cycyclopropane synthesis.