Halohydrin Formation from Alkenes
HALOHYDRIN FORMATION FROM ALKENES
Halohydrins is a functional group in which a hydroxyl group and a halogen groups are attached to adjacent carbon atoms. Halohydrins are good precursors for a number of polymers. They may be classified as bromohydrin, chlorohydrin, or iodohydrin depending on the halogen group attached.
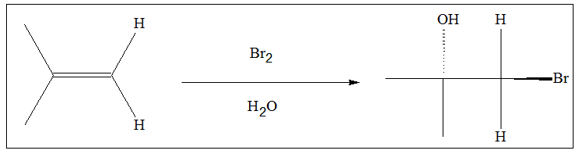
Halohydrins are usually synthesized by treating an alkene with a halogen, in presence of water. This is a type of electrophilic addition with mechanism similar to that of halogenation, which involves formation of a cyclic intermediate. Eventually, water is added in anti position. The common reaction for halohydrin formation is shown below.
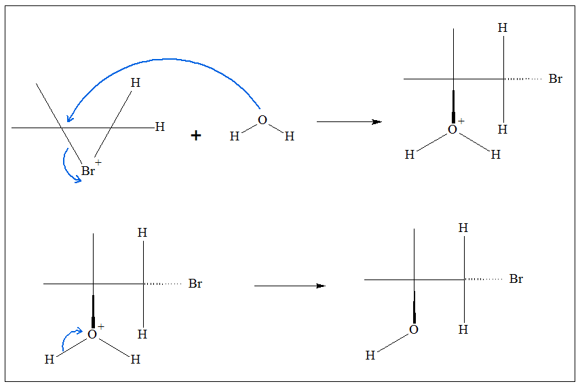
Halohydrin formation proceeds by a two-step process. The detailed mechanism is as follows:
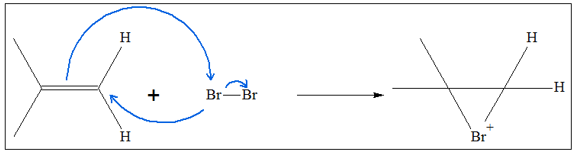
- 1. The first step of the reaction is addition of the halogen through the C=C. Just like in halogenation, one of the X atom forms a cyclic intermediate with the C=C carbon. This is possible because the C=C can donate an electron pair to one of the X atom, while the X atom can also donate an electron pair to one of the carbon atoms leading to formation of the bridged intermediate.
- 2. Because of the bulkiness of the intermediate, the only point of attack for the water molecule is through the backside of the intermediate. This means water is added anti to the halogen group. When water is added to one of the carbon atoms, the cyclic chain opens in such a way that the halo group remains attached to the other carbon atoms.