Free Radical Addition of HBr
FREE RADICAL ADDITION OF HBr
Alkyl halides can be produced by a variety of mechanisms. One way of producing the alkyl halides is the treatment of alkenes with acids. Normally, this process proceeds via an electrophilic addition mechanism. However, in presence of organic peroxides, hydrogen bromide (HBr) adds to the alkene group through a different mechanism.
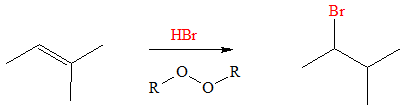
Normally, when treated with HBr, alkenes produce a Markovnikov Product. This is because in electrophilic addition, a carbocation is formed and rearrangements, or mechanism itself, enables production of a Markovnikov Product. However, in presence of peroxides, the peroxide facilitates a different mechanism for the HBR to be added to the alkene group. The reaction proceeds via formation of radical intermediates.
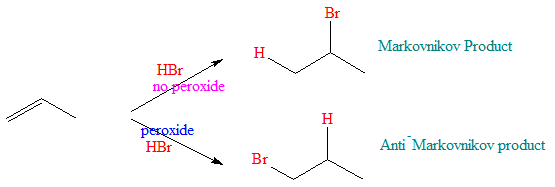
With the organic peroxide present, a free radical chain reaction occurs when HBr is added to alkenes. The steps in the mechanism for this reaction is shown below:
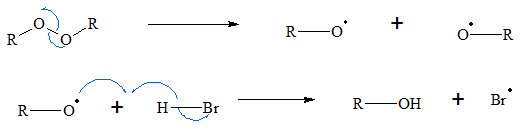
- 1. The chain is initiated by organic peroxide breaking forming free radicals. The oxygen to oxygen bond is broken down to produce the free radical which will attack the HBr molecule.
The free radical will then attack one of the olefinic carbon forming the brominated free radical.
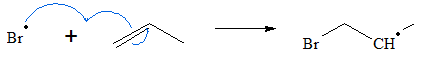
- 2. Since free radical are still present in the solution, the free radicals continue to react with other HBr molecule to form more free radicals which can attack other molecules present.

- 3. When two free radicals hit each other, the process stop because there is no new free radical formed.
The problem with free radical reactions is the high possibility of producing mixed product. If we want to produce a monobrominated alkane, it will be hard to achieve in presence of peroxides. Unless, we are able to terminate immediately the given reaction, the polymer will continue to grow.
Free radical chain reactions are extremely helpful in producing different polymers. This is because the mechanism of formation can be used in chain elongation. For example, when a alkyl free radical attacks another alkene compound, it leads to formation of a longer free radical. Unless terminated, the chain will continuously grow.
