Halogenation of Alkenes
HALOGENATION OF ALKENES
Another important addition reaction of alkenes is halogenation. Halogenation involves the addition of X2 (where X2 can be Br2 or Cl2) to an alkene. In the process, each of the carbon atoms in the double bond forms new sigma bonds with one X atom each. An example of halogenation reaction is shown below.
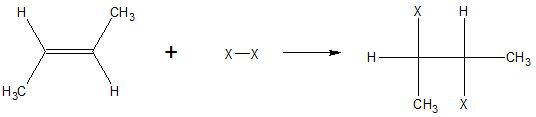
The process is only applicable to chlorine and bromine because of the relatively more convenient reaction conditions and yield. The fluorination reaction is not usually done because it is too violent. On the other hand, Iodination produces very low yields.
In halogenation reaction, the two halogen atoms attach themselves on opposite sides of the pi bonds. This mode of addition is termed as anti addition. Because of the addition on opposite sides of the halogen atom, this type of reaction usually produces a chiral molecule.
Compared to hydrohalogenation reaction, the mechanism for the halogenation reaction is more complicated because of the need to produce an intermediate that would only allow addition on a specific side of the carbon-carbon double bond. A typical carbocation intermediate, just like that in hydrohalogenation, can accept an incoming nucleophile atop or below the original pi bond. This means a normal free carbocation cannot be produced as an intermediate in halogenation reaction because it still allows production of dihalides where the halogens are on the same side of the carbon chain.
A more plausible mechanism for this reaction is the formation of a bridged intermediate. In the case of bromination reaction, a bromonium ion intermediate is produced. An example of a bromonium intermediate is shown below.
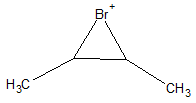
Because of having a bulky group atop of the original C=C, the only point of attack left for an incoming nucleophile is at the bottom of the chain. This back-side attack enables production of the anti-product. For example, when 2-methylbut2-ene is treated with Cl2, a chloronium intermediate is first produced leaving a free Cl- ion. The chloride ion, being a relatively big species, it does not tend to attack at the same side as the cyclic part of the intermediate molecule. The route that will require less energy due to having less bulky group hindering the attack is through the back-side of the intermediate. When the Cl- ion attaches itself to one of the vinyllic carbons, the cycle opens up with the Cl atom in the cyclic intermediate remains attached to the other vinyllic carbon. The detailed mechanism for this reaction is shown below.

