Synthesis of Alkenes
SYNTHESIS OF ALKENES
Alkenes can be prepared from a variety of reagents. Common sources of alkenes are alcohols and alkyl halides. In terms of mechanism, preparation of alkenes involves removal of one group from two adjacent carbon atoms. In the process, two sigma bonds are lost and is replaced by a pi bond between two carbon atoms. These reactions are called elimination reactions.
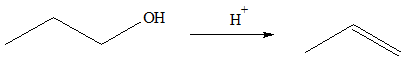
Elimination reactions proceed either via an E1 mechanism or an E2 mechanism. The difference between these two mechanisms is the molecularity of the rate-determining step, which is the first step of the reaction. E1 reactions have rate-determining steps that are unimolecular. The mechanism involves removal of one group, producing a carbocation intermediate. To stabilize the carbocation, a hydrogen atom from adjacent carbon will be released and a pi bond between this carbon and the charged carbon will be formed. E2 reactions, on the other hand, have bimolecular rate-determining step. Here, removals of groups from 2 adjacent carbon atoms, as well as, the formation of the pi bond occur simultaneously. No carbocation intermediate is formed in this mechanism.
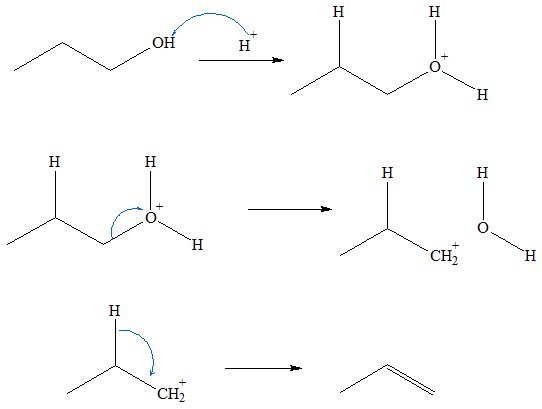
Alkenes can be prepared dehydration of alcohols. In the process of the reaction, a water molecule is released from the alcohol. A strong acid catalyzes the dehydration process. The reaction proceeds via an E1 mechanism. The first step in this reaction is the protonation of the –OH group to produce a charge aqua group. Because of the relative instability of an oxygen atom to have a positive charge, there is a high tendency for the water molecule to be released from the structure and produce a carbocation intermediate in the process. Because the carbocation is a very unstable species, a water molecule can abstract a hydrogen atom from a carbon adjacent to the charged carbon atom. The release of the H atom will facilitate formation the pi bond. The end products for this reaction are protonated water and alkene. In terms of reactivity towards dehydration, tertiary (3o) alcohols are the most reactive while primary (1o) alcohols are the least reactive. This is because tertiary alcohols produce more stable carbocations than primary alcohols.
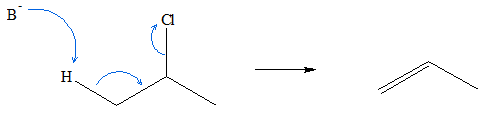
Alkyl halides can also be used to produce alkenes. Synthesis of alkenes proceeds via a process called dehydrohalogenation. It is termed as such because a hydrogen atom and a halogen group is removed in the process. This specific reaction follows an E2 mechanism. In this reaction, a strong base is needed to abstract the proton from a carbon atom adjacent to the C-X group. A transition state is formed in the process where bond formation between the base and the hydrogen atom to be abstracted, occurs simultaneously with the bond-breaking between the C-H bond, formation of C-C pi bond, and bond breaking of C-X bond. When the hydrogen atom and the halogen group is released completely, the end-product will be an alkene.