Properties of Alkenes
PROPERTIES OF ALKENES
Alkenes are organic compounds, which contain at least one carbon-carbon double bond (C=C) in it structure. These compounds are considered unsaturated because there are carbon atoms which are not fully substituted, or do not have four atoms directly attached to it. These compounds differ from alkanes by having two less hydrogen atoms for every double bond present. The presence of the C=C groups affects both the physical and chemical properties of alkenes in comparison to that of alkanes.
Figure 1. Structures of Ethane and Ethene.
Just like its alkane counterparts, alkenes are hydrophobic compounds, which means they cannot be dissolved in water. This hydrophobic nature of the compound is due to it being a non-polar compound. Because of the closeness in the electronegativity values of carbon and hydrogen, the bonds formed by these atoms are non-polar. Alkenes are only soluble in non-polar solvents.
Also because of its non-polar nature, the type of intermolecular forces of attraction present in these compounds is just London Dispersion Force (LDF). Since LDF is a very weak type of force between interacting molecules, separating one molecule from another will be very easy. This is apparent in the melting and boiling points of compounds. Table 1 below summarizes the melting point and boiling points of some alkenes.
Table 1. Melting and Boling Points of Common Alkenes
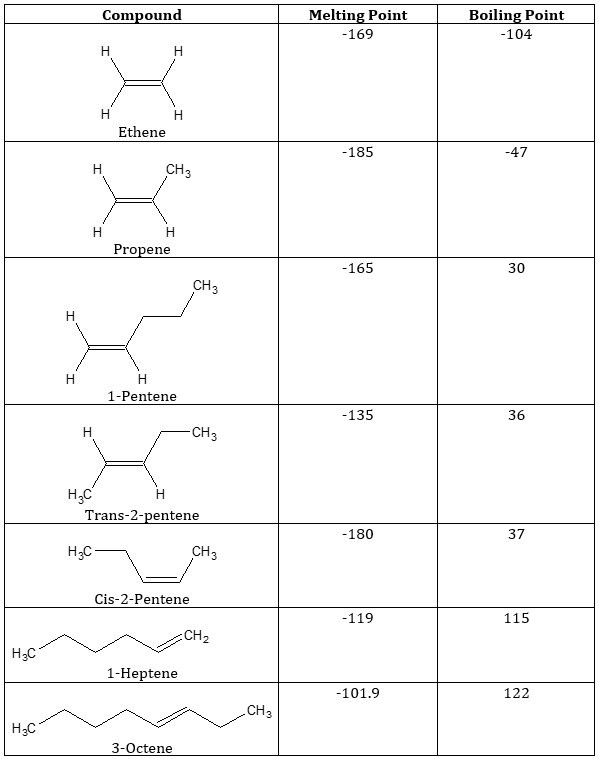
As shown in the table, the general trend for both the melting point and the boiling point is, the longer the length of the carbon chain, the higher are the values for the two properties. This trend is due to having stronger LDF as the molecules becomes larger. The larger the molecule, the more polarizable it becomes and so the stronger is the LDF. Stronger LDF means it is harder to melt or boil these compounds.
Structural configurations of compounds also affect the physical properties of compounds. For geometric isomers, the cis isomer has the higher boiling point, while the trans isomer generally has the higher melting point. The higher melting point of trans isomers is because of the better packing of trans isomer molecules due to its structure. Ordered packing is easier to achieve for trans than cis configuration. The higher boiling point of cis isomer is due to the relative polarity of the compound. Methyl groups tend to push electrons away from it, towards the double bond. In trans configuration, the relative polarity of the bonds cancels out because of their opposite directionality. As compared to cis molecules where in both sides of the double bonds, the direction of polarity is the same and so they do not cancel out. This relative polarity of cis isomers enable it to have a lower boiling point.
