Hydrohalogenation of Alkenes
HYDROHALOGENATION OF ALKENES
Alkenes are compounds that contain at least one carbon-carbon double bond. Because of the reactivity of the pi bonds within the structure of the compound, alkene can undergo a variety of addition reactions. One of the most common addition reactions for alkene is hydrohalogenation.
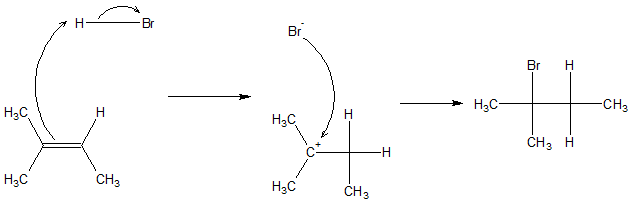
Hydrohalogenation is a type of addition reaction of alkene where an H and X atom is added to the unsaturation site on the alkene structure. The process involves reacting alkene with HX (where X = Cl, Br, or I). The H and X are added across the pi bond. An example of this process is shown below.
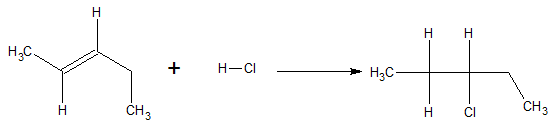
The process of addition proceeds by firs having the proton attack one of the carbons of the pi bond. Because of these, electron rearrangement leads to production of a carbocation. The carbocation is then attacked by the negatively charged anion (X-). The specific mechanism for the reaction is shown below.
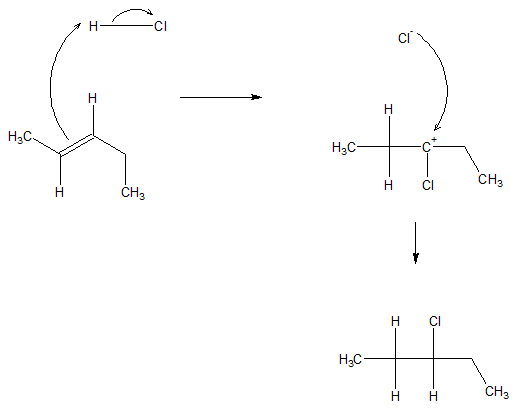
Hydrohalogenation reaction is relatively simple for symmetrical alkenes. However, there are special considerations for asymmetrical alkenes. In 1869, Vladimir Markovnikov, developed a rule applied to addition reactions based on his observations. The rule states that the hydrogen atom attaches itself to the less substituted carbon (vinyllic position containing the larger number of hydrogen atoms). The X atom on the other hand is attached to the more substituted carbon.
Following Markovnikov Rule, it is observable that it involves formation of the more stable carbocation. Because of having a more stable carbocation, the process of adding the nucleophile X- will require less energy. The lower energy barrier of formation of a tertiary carbon compared to a secondary carbon results to a faster reaction.