Stereochemistry of Alkenes
STEREOCHEMISTRY OF ALKENES
Studying the spatial orientation of a molecule is important because of its effect on the different physical and chemical properties on the molecule. Just like alkanes, alkenes also exhibit a number of stereoisomers. Specifically, alkenes exhibit multiple geometric isomers. Geometric isomers are non-superimposable and non-mirror image of the same compound.
In alkenes, instead of looking into only one carbon atom, what is studies is the environment around the two carbons consisting the double bond. If there are exactly two substituents and two hydrogen atoms attached to the C=C, the isomers may be labeled as cis- or trans-.
The cis- and trans- configuration differs in the arrangements of the substituents around the C=C. Cis isomers are alkenes whose substituents with the highest priority are on the same side of the carbon-carbon double bond. On the other hand, the trans- isomers are alkenes whose substituents with the highest priority is on different sides of the carbon-carbon double bond. Below are examples of the cis- and trans- isomers of 2-butene.
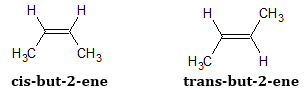
In identifying the group of the highest priority around the C=C, the Cahn-Ingold-Prelog Priority System is employed. The specific rules in identifying if the substance is cis or trans are as follows.
- 1. In each of the sp2 hybridized carbon, assign the priority numbers (1 = high, 2 = low) based on the atomic number of the element directly attached to the sp2carbon. The substituent with the higher atomic number will be given a higher priority.
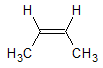
For each carbon atom in the double bond, one H and one C atom is directly attached as substituents. Since C has a higher atomic number than H, the methyl substituent is given a higher priority than the H.
- 2. When the two atoms directly attached to the same sp2 carbons are isotopes of each other, the heavier isotope will be given a higher priority.
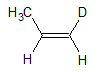
The deuterium substituent is given a higher priority than a hydrogen substituent because D is a heavier atom.
- 3. In cases where the same atom is attached, priority level is determined by moving away from the C=C, and looking for the first point of difference. When the first point of difference is identified, the priority rule applies again.
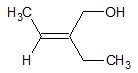
For the double bonded carbon on the right, both substituents involve a carbon atom directly attached to the double bonded carbon. Since this is the same, we move one more atom away from the double bond. For the methyl group, there are three hydrogen atoms after the C that is directly attached. For the hydroxymethyl substituent, there are two H and one O atom attached to the C atom directly bonded to the double bonded carbon. This is the first point of difference. Since O has a higher atomic number than H, the hydroxymethyl substiuent is given a higher priority than the methyl substituent.
The cis/trans system is only used when an alkene has two different groups on each carbon atom of the double bond and each carbon has one of the same group. However, there are alkenes where none of the substituents around the C=C are the same. In this case, the E/Z system will be used.
In the E/Z system, the Cahn-Ingold-Prelog Rules still applies. To determine if the compound is E or Z, the following steps are followed.
- The alkene is split perpendicular to the double bond.
- Using the Cahn-Ingold-Prelog System, the relative priorities are then assigned to the substituent in each of the carbons in the double bond.
- The positions of the substituent with higher priority are then compared.
- a. If the higher priority groups are on the same side, the compound is labeled Z.
- b. If the higher priority group is on opposite sides, the compound is labeled E.
For example, in the structure below, there are four different substituents around the C=C. The order of priority for the carbon on the right was already determined. For the carbon on the left, C and H are directly attached to the double bonded carbon atom. In this case, the one assigned with higher priority is the methyl group than the H group. Comparing the positions of the two substituents of higher priority, it can be said that both are on top of the C=C. Since these two group are on the same side, the compound is labeled Z. The name for the compound below is (Z)-2-ethylbut-2-enol.
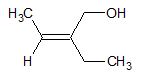
- 4. If there are more than one double bond present in the structure, the position of the double bond is indicated in the name of the compound.
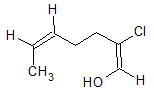
Since there are two double bonds in the compound, each of these double bonds should be labeled as either E or Z. For the double bond on the left, the higher priority groups with both C atoms directly attached to the double bonded carbons are on the same side, it should be labeled Z. For the double bond on the right, the higher priority groups are Cl and OH. These two groups are on opposite sides of the double bond and so the C=C is labeled E. The name of the compound is (1E,5Z)2-chlorohepta-1,5-dienol.
