Hydroboration of Alkenes
HYDROBORATION OF ALKENES
Alcohols can be prepared by a number of possible reactions. These include acid-catalyzed hydration, oxymercuration and hydroboration. The focus of this article is hydroboration. Hydroboration is named as such because a hydrogen atom and a boron group are added across the pi bond. This procedure was developed by H.C. Brown and gave him the Nobel Prize in 1979.
The mechanism for hydroboration is not as simple as the acid-catalyzed hydration. This involves a multistep mechanism that produces a regioselective product. Another difference between hydroboration and the two other prcedures to produce alcohol is the position of the –OH group in the final structure. Hydroboration does not follow the Markovnikov’s Rule that means the H atom is added to the more substituted carbon. This is because of the spatial consideration in the addition process.
The specific steps in hydroboration are as follows:
- 1. Borane (BH3) is added through the pi bond. The addition of borane does not follow the Markovnikov’s Rule. Hydrogen is added to the more substituted carbon. The addition follows production of a transition state that involves simultaneous bond breaking of H from BH3, bond making between B and C, bond making between an H and a C, and bond-breaking of the C=C pi bond. Below is a sample of the reaction described.
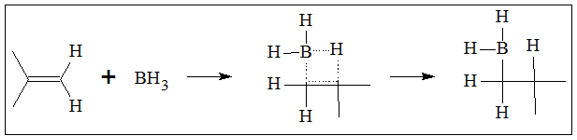
The boron atom is added to the less substituted carbon because of steric factors. The addition of the large BH2 group is favorable to the less congested end of the alkene. Since there is no carbocation formed, rearrangement in the structure is not possible.
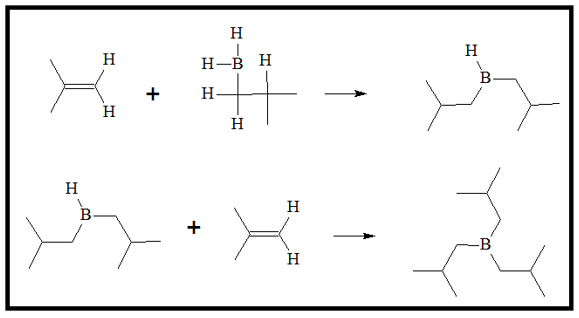
Because of the formation of the 4-sided transition state, the addition of BH2 and H atom are on the same side. This mechanism does not allow formation of intermediate as it does not allow rotation of the C-C single bond. The process of addition of alkenes continues to produce a fully substituted alkyl borane.
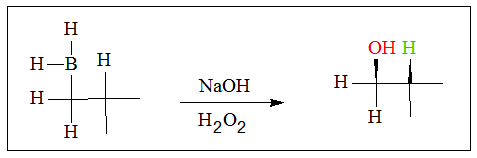
- 2. When alkyl borane is reacted with hydrogen peroxide, boric acid and an alcohol is produced. The mechanism for alcohol formation is a repeated process of addition of peroxidate anion (HOO-). The filled orbital on oxygen reacts with the empty 2p orbital on boron. This process is a type of nucleophile-electrophile reaction.
Having a free p orbital in the boron atom enables it to act as an electrophile. It can readily receive electrons from the peroxidate ion. The negatively charged intermediate is produced in the process.
- 3. An alkyl group then transfers to the O atom attached to the boron atom, while an OH- group is released to produce a neutral molecule. This process is repeated for two more times to produce the boronic ester.
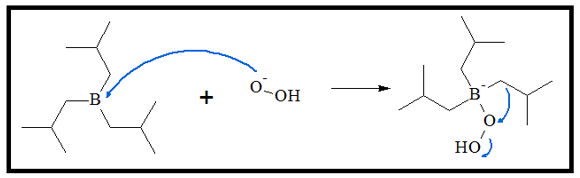
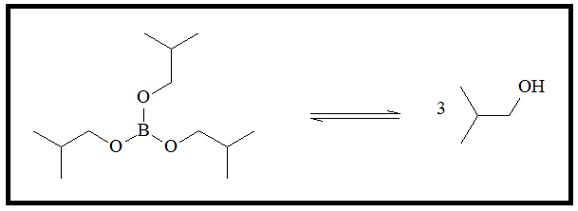
With excess amounts of the peroxide ion, the fully substituted boronic ester is produced.
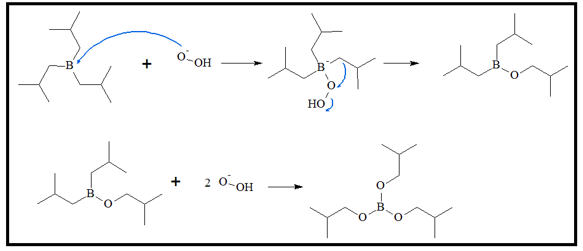
- 4. When there are excess hydroxide ion, the boronic ester equilibrates with boronic acid and the alcohol.