Properties of Amines
Properties of Amines
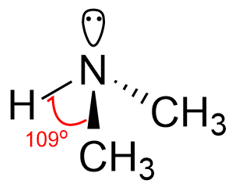
Amines are organic derivatives of ammonia in which or more alkyl or aryl groups are bonded to the nitrogen atom. The nitrogen atom in amines is sp3 hybridized with regular tetrahedron geometry with C-N-C bond angle of about 109o.
Amines are polar in nature. Both the N-H and C-N sigma bonds are polar due to electronegativity differences. Primary and secondary amines containing N-H bonds tend to form hydrogen bonds. While tertiary amines containing no N-H bond cannot form hydrogen bonds although, tertiary amines can make hydrogen bonds with other molecule containing F-H, N-H or O-H groups. The nitrogen atom of amines can always act as hydrogen bond acceptor.
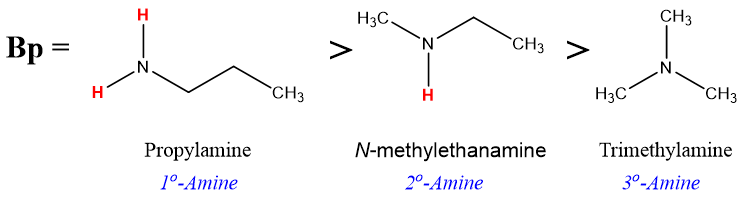
Amines with lower aliphatic carbon chains are gases at room temperature and they have characteristic odor of rotting fish. Primary amines containing carbon chain of three or four carbons are liquids at room temperatures. While amines with greater number of carbon atoms are solids at room temperatures. The N-H bond of amines is less polar than the O-H bond of alcohols. This results in a lower boiling point of amines compared to alcohols of similar molecular weights. For example, propyl amine (MW = 59 g/mol) has a boiling point of 48 °C whereas, propanol (MW = 60 g/mol) has a boiling point of 97 °C.
On the other hand, primary amines have higher boiling points than secondary and tertiary amines. For example, boiling points of isomers of amines with primary, secondary, and tertiary structures are listed below.
All primary, secondary, and tertiary amines are soluble in polar protic solvents. Almost all amines are soluble in alcohols while, amines with carbon chain length of upto six are also soluble in water. The solubility of amines in water decreases with increase in carbon chain length. The increase in carbon chain length increases the hydrophobic character of amines thus making it insoluble in water. Following are some examples of solubilities of different amines in water.
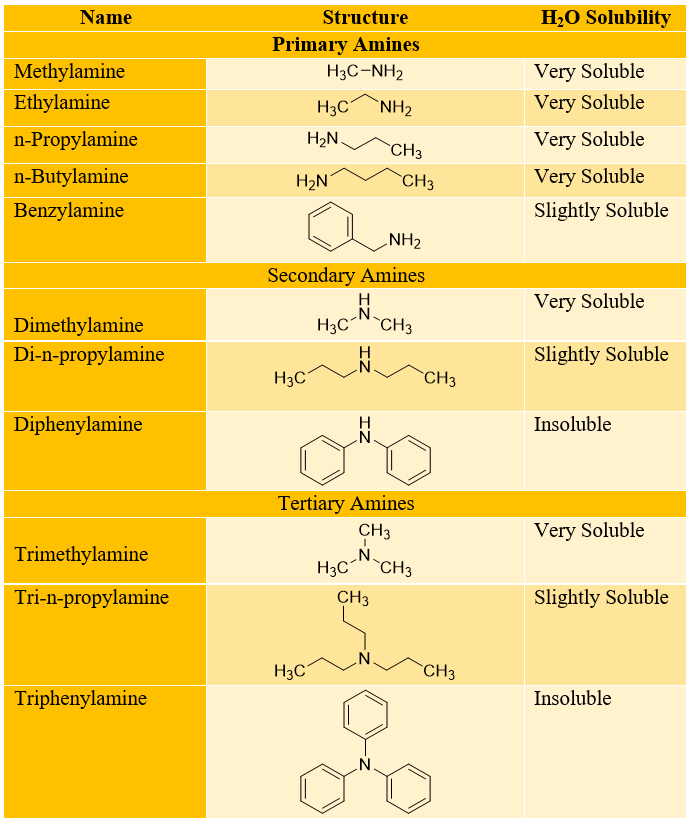
Hence, the solubility of amines in water depends on N-substitution and number of carbon atoms present in the molecule. The greater the number of N-H bonds the the greater is the solubility of amines in water and vice versa and the smaller the number of carbon atoms the greater is the solubility in water and vice versa.
Basicity of Amines:
Amines are basic in nature due to the presence of lone pair of electrons on nitrogen atom. Amines reacts with acids to form corresponding salts. Furthermore, amines also act as nucleophiles and react with different electrophiles in polar reactions. Following are some factors that effects the basicity of amines.
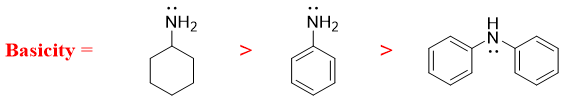
- a) The basicity of amines increases with an increase in the negative charge. The greater the negative charge the greater is the basicity and vice versa. For example,

- b) Those amines in which the lone pair of electrons on nitrogen atom conjugate with the side chain pi bonds due to resonance are less basic in nature. This is due to the unavailability of lone pair electrons as they are busy in resonance. For example,
- c) The presence of electron withdrawing groups on amines decreases the basicity due to negative inductive effect. The electron density is hogged off from the nitrogen atom hence, making it difficult to donate. For example,
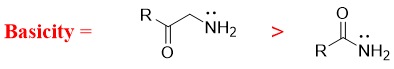
- d) Amines in which the nitrogen atom act as a π-donor are less basic in nature. For example, in amides the nitrogen atom is bonded to the carbonyl group. The nitrogen atom donates its lone pair of electrons to carbonyl group and forms new π bond. In this case the carbonyl oxygen acts as π-acceptor and obtains negative charge.
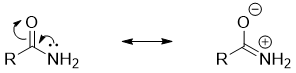
Once the nitrogen atom forms π-bond it cannot act as base anymore. Thus, the basicity decreases when nitrogen atom has ability of making π-bond.
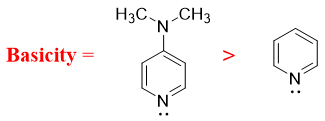
- e) Amines in which the nitrogen atom act as a π-acceptor are more basic in nature. For, example, the -N(CH3)2 group at para position in 4-dimethylamino pyridine act as a π-donor and pyridine nitrogen act as π-acceptor. The resonance results in the movement of electrons from -N(CH3)2 group to nitrogen atom as shown below.
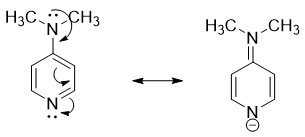
Hence, when nitrogen atom of amines acts as π-acceptor then it has higher basicity.
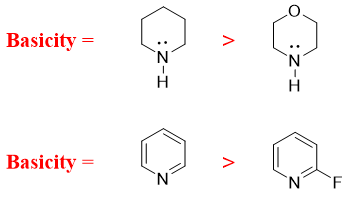
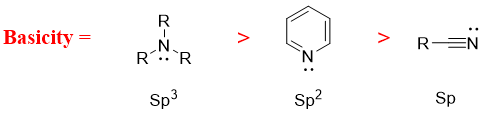
- f) Next factor effecting basicity of amines is hybridization. The smaller the s character the greater is the basicity of amine. This is because the s orbital is closer to the nucleus hence, the lone pair of electrons on nitrogen observes more positive character and hence making it less basic.
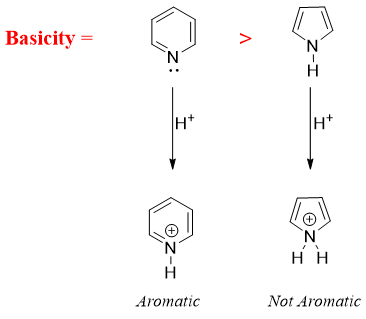
- g) Cyclic amines in which the ring system is aromatic have different basicities. Those amines which maintains its aromaticity after protonation are more basic. For example,