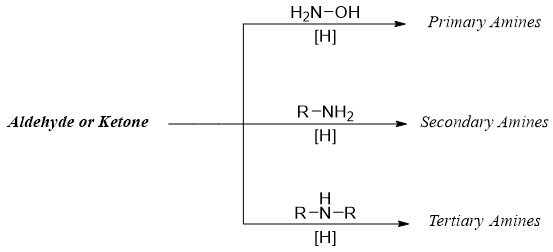
Synthesis - Reductive Amination
Synthesis - Reductive Amination
Aldehydes or ketones when reacted with ammonia or amines produced corresponding imines. The imines formed are further reduced to amines by using reducing agents. This overall process is called reductive amination.
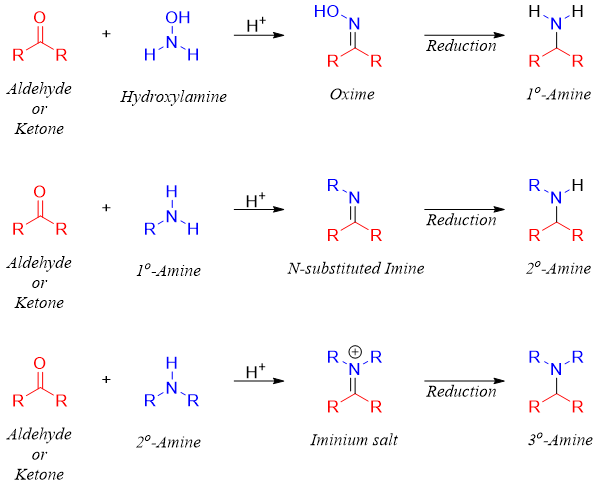
The synthesis of primary amines is generally carried out via oxime intermediate. Oximes are much stable than imines formed by ammonia. The oximes can be reduced to primary amines by using different reducing reagents including LiAlH4 and Zn and HCl.
Initially the mechanism involves the formation of imine from the reaction of aldehyde or ketone with amines. This reaction is acid catalyzed.
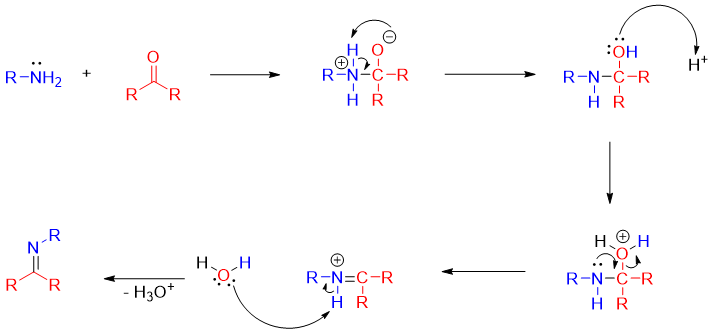
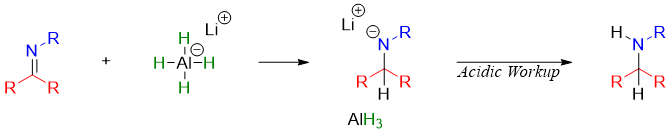
The reduction of imine is carried out by using different reducing reagents. Following mechanism shows the reduction of imine by NaBH4 or LiAlH4.
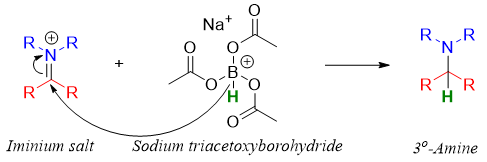
For the synthesis of tertiary amines, aldehydes and ketones are reacted with secondary amines. This results in the formation of iminium salts. Iminium salts are unstable and cannot be isolated therefore, they are reduced in situ. In this case reducing reagents like NaBH4 and LiAlH4 cannot be used because they can reduce aldehydes and ketones to alcohols. To overcome this problem sodium triacetoxyborohydride (Na(CH3COO)3BH) is used as a source of hydride ion. Sodium triacetoxyborohydride is less reactive than NaBH4 thus, they selectively reduce the imine functionality.
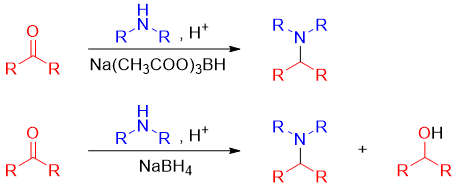
Following step shows the addition of hydride ion to iminium salt.
The reaction of ketones with secondary amines can also result in the formation of an enamine. The enamine is also reduced by above mentioned reducing reagents. Sodium triacetoxyborohydride is commonly used as a reducing reagent because it is not toxic like its older version sodium cyanoborohydride (NaBH3CN) and it is easily handled and it is stable even in acidic conditions.
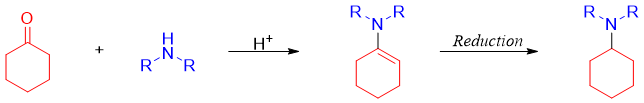
Thus, the reductive amination reaction can be utilized to produce primary, secondary, and tertiary amines from aldehydes and ketones.