Mannich Reaction
Mannich Reaction
Mannich reaction is a three component reaction. It involves the condensation of carbonyl compound (enolizable, containing acidic α-hydrogen) with aldehyde (non-enolizable like formaldehyde) in the presence of ammonia, primary amine, or secondary amine to produce β-aminocarbonyl compound called Mannich base.
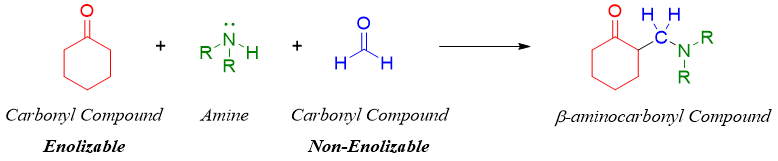
The reaction involves the formation of Schiff base or iminium ion by the reaction of amine and formaldehyde.
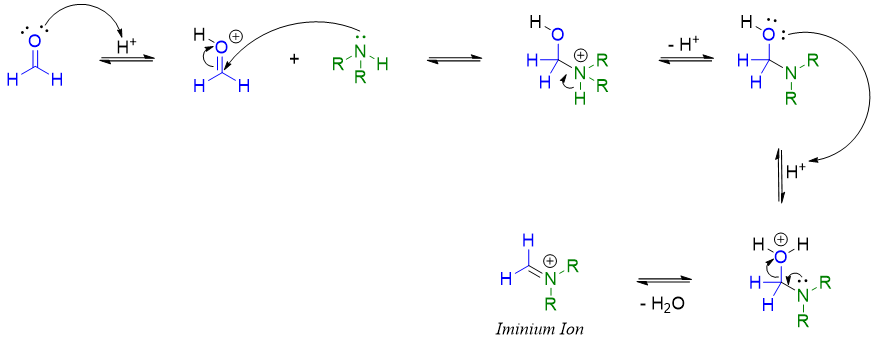
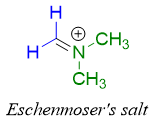
The iminium ion containing two methyl groups om nitrogen atom is called as Eschenmoser's salt.
The second carbonyl compound containing alpha acidic proton can tautomerize to form an enol.
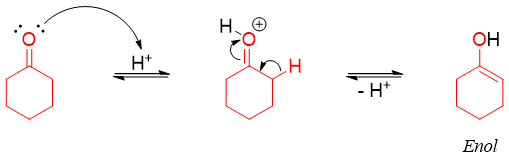
The enol formed attacks the iminium ion.
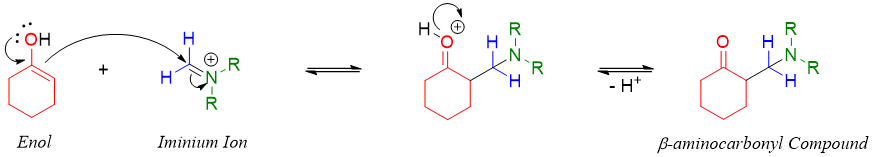
The Mannich product β-aminocarbonyl compound can further be converted into enones. The enones are synthesized by first reacting β-aminocarbonyl compound with methyl iodide (CH3-I) and then reacting the resulting ammonium salt with base.
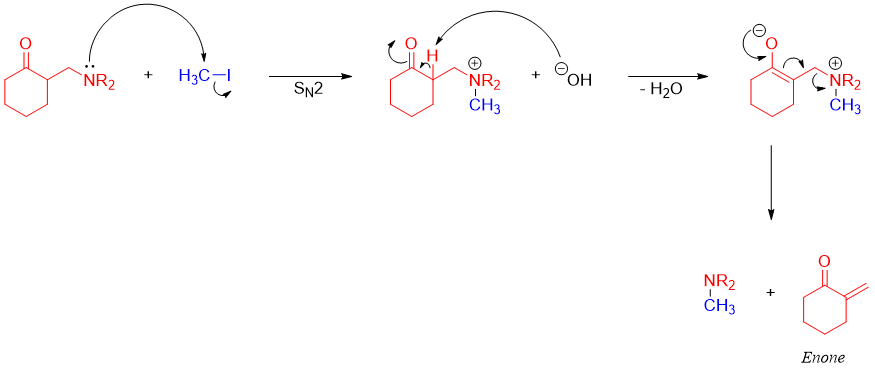
The enones formed above are exo-methylene compounds. They are very reactive and not easily formed or stored. Enones cannot be formed by simple aldol condensation reaction because aldol condensation reaction will produce many side products as the formaldehyde (also known as super aldehyde) is extremely reactive thus, giving off many unwanted products.
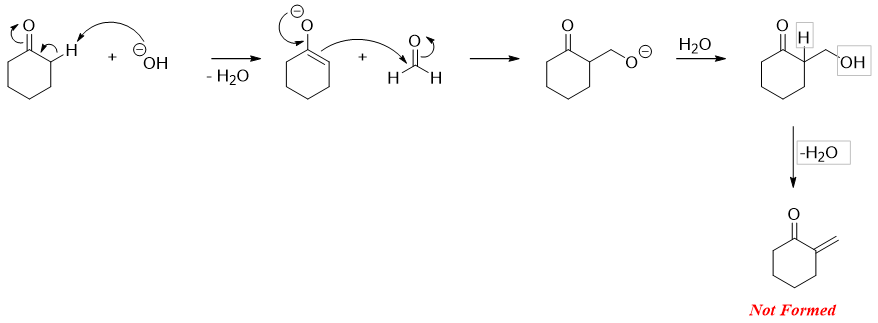
The exact aldol reaction between formaldehyde and carbonyl compound containing alpha acidic proton is given below.
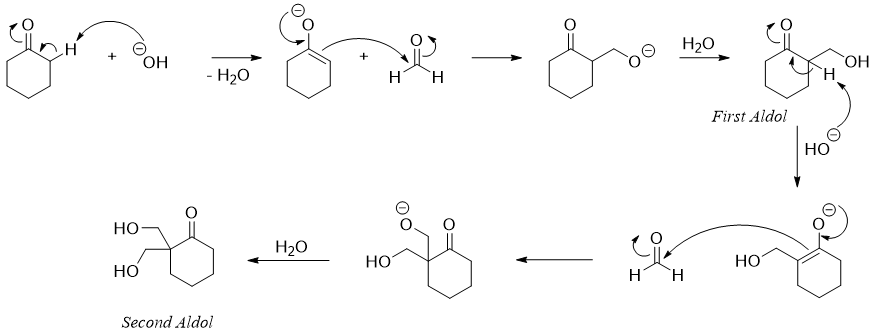
The greater the number of alpha acidic protons the greater will be the number of aldol products in aldol condensation reaction.
Mannich reaction has many applications including synthesis of natural products i.e., antibiotics, alkaloids, nucleotides, peptides etc. This reaction is also employed in the synthesis of agriculture chemicals including plant growth regulators. Many medicinal drugs are synthesized via Mannich reaction i.e., fluoxetine (anti-depressant), rolitetracycline (base for tetracycline), tolmetin (anti-inflammatory) etc.
