Amines as Bases
Amines as Bases
Like ammonia, primary, secondary, and tertiary amines contain a lone pair of electrons on the nitrogen atom. This lone pair of electrons can be donated to a proton or any electron deficient atom thus, making the amines basic in nature.
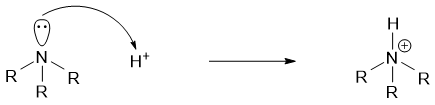
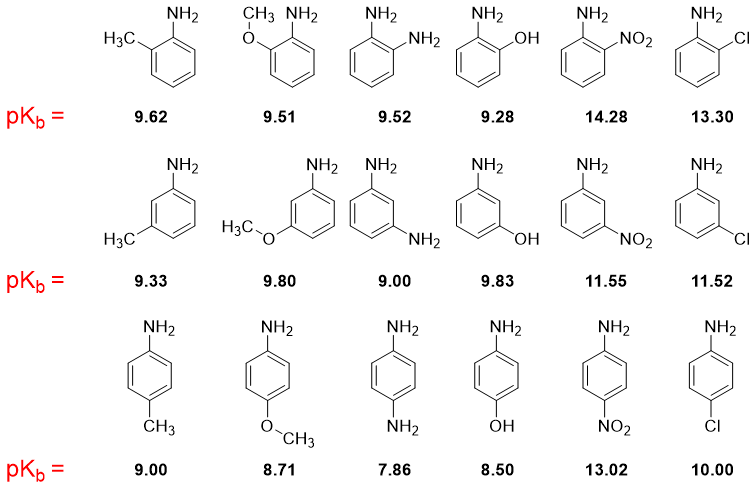
The basicity of an imine is measured in basicity constant Kb. Kb measures the extent of an amine to accept a proton. The greater the Kb value the greater is the basicity of an amine and vice versa. The negative logarithm of Kb is pKb. The smaller the pKb value the stronger is the basicity of an amine and vice versa. Following are some pKb values of different amines.
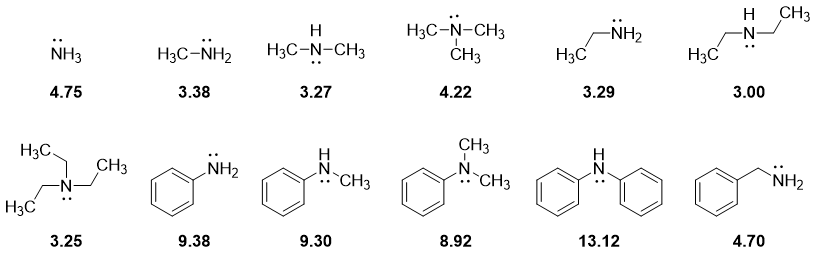
From above pKb values of amine derivatives following conclusions can be made.
i) The aliphatic amines are more basic than ammonia.ii) The aromatic amines are less basic than ammonia.
iii) N-substituted aromatic amines are slightly more basic than pure aromatic amines.
Primary, secondary, and tertiary amines are more basic than ammonia because of the electron donating alkyl groups. The greater the number of alkyl groups the greater will be the basicity of alkylated amines. The electron donating groups increases electron density on nitrogen atom thus, making it easier to donate the lone pair of electrons.
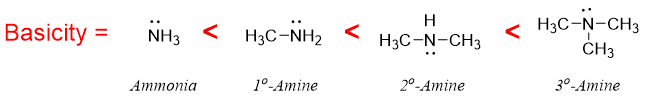
In aqueous solutions, the basicity of all three classes of amines changes. The new basicity order in aqueous solutions is as following.
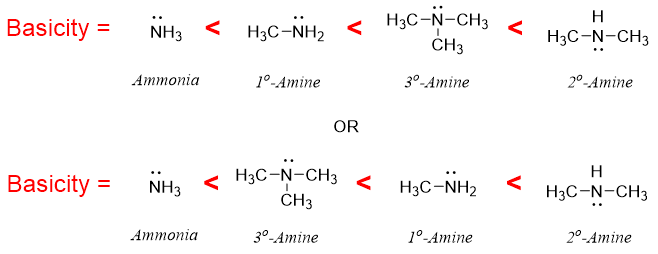
In aqueous solution secondary amines are the strongest bases while the tertiary amines are less basic than secondary amines followed by primary amines. In some cases, tertiary amines get even weaker than primary amines. The change in basicity order in aqueous solution arises due to the stability of conjugate acid or ammonium cation. The stability of conjugate acid depends upon following factors.
- a) Inductive effect of Alkyl groups: With increase in the number of alkyl groups, the dispersal of positive charge on conjugate acid increases due to positive inductive effect of alkyl groups. Thus, due to inductive effect the basicity order is as follow.
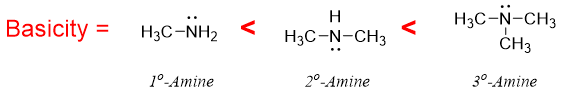
- b) Magnitude of Hydrogen bonding with water: The greater the number of hydrogen atoms present on nitrogen atom on ammonium cation the greater is the number of hydrogen bonding with water molecules and the greater is the stability of ammonium cation. Therefore, on the basis of hydrogen bond interactions with water tertiary amines are the least stable amines and therefore least basic in nature.
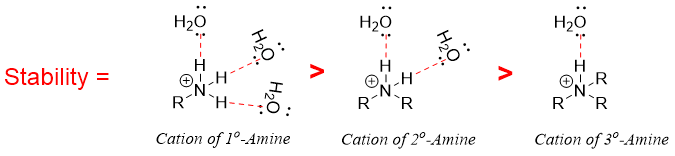
- c) Steric effect of Alkyl groups: In ammonium cations of tertiary amines, due to steric repulsion the extent of hydrogen bonding further decreases thus making it more unstable and least basic.
Combining the above three factors, secondary amines tent to be more basic than primary and tertiary amines in aqueous solutions. Furthermore, the basicities of primary and tertiary amines in aqueous solutions are variable. The bulkier the alkyl group the smaller the hydrogen bondings and the lesser the stability and vice versa.
Aralkylamines have aromatic ring attached to the carbon atom to which nitrogen atom is bonded. The aromatic rings have negative inductive effect thus they can decrease the electron density on nitrogen atom making it less basic. The greater the distance between aromatic ring and amino group, the greater is the basicity of aromatic amine and vice versa.

On the other hand, aromatic amines are less basic than aliphatic amines and ammonia. This decrease in basicity is due to resonance effect. The lone pair on nitrogen atom get delocalized over the benzene ring thus, making it less available to be donated. The greater the number of benzene rings attached to nitrogen atom the smaller is the basicity of corresponding amine and vice versa.
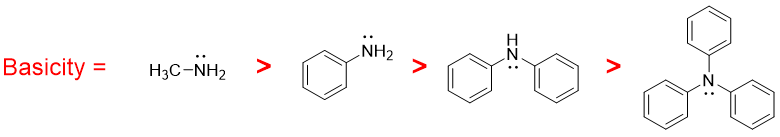
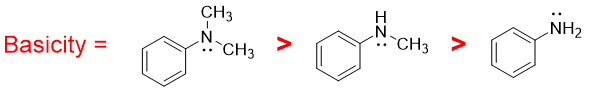
When the hydrogen atom in primary aromatic amines is replaced by alkyl group, it increases the electron density on nitrogen atom thus making it more basic,
The presence of electron donating substituents on benzene ring increases the basicity while, electron withdrawing substituents on benzene ring decreases the basicity. These substituents are more effective if present on -meta and -para positions. The -ortho substituted (either withdrawing or donating groups) aniline derivatives are less basic due to ortho effect.
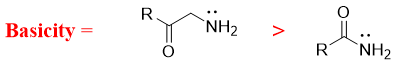
Amines in which the nitrogen atom act as a π-donor are less basic in nature. For example, in amides the nitrogen atom is bonded to the carbonyl group. The nitrogen atom donates its lone pair of electrons to carbonyl group and forms new π bond. In this case the carbonyl oxygen acts as π-acceptor and obtains negative charge.
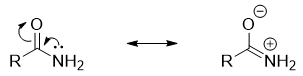
Once the nitrogen atom forms π-bond it cannot act as base anymore. Thus, the basicity decreases when nitrogen atom has ability of making π-bond.
Amines in which the nitrogen atom act as a π-acceptor are more basic in nature. For, example, the -N(CH3)2 group at para position in 4-dimethylamino pyridine act as a π-donor and pyridine nitrogen act as π-acceptor. The resonance results in the movement of electrons from -N(CH3)2 group to nitrogen atom as shown below.
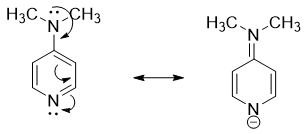
Hence, when nitrogen atom of amines acts as π-acceptor then it has higher basicity.
