Nomenclature of Amines
Nomenclature of Amines
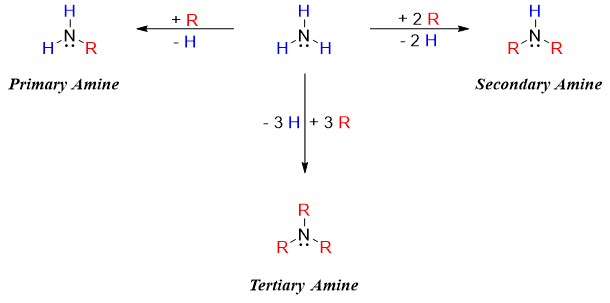
Amines are organic derivatives of ammonia in which at least one hydrogen atom is replaced by an alkyl or aryl group. Depending upon the number of alkyl or aryl groups bonded to nitrogen atom, amines are classified as primary, secondary and tertiary amines.
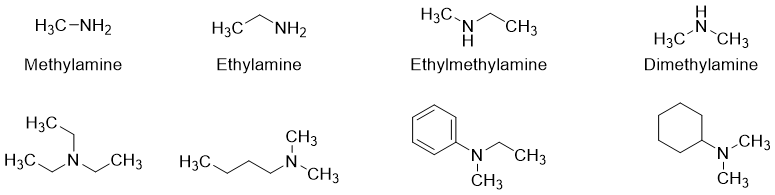
Common naming of Amines:
Amines are commonly named by naming the alkyl groups attached to the nitrogen atom in alphabetical order followed by the word “amine”. For example,
The common names of quaternary amines consist of the name of alkyl groups in alphabetical order followed by “ammonium” (no space between ammonium and alkyl name) and at the end “anion” name is assigned. For example,
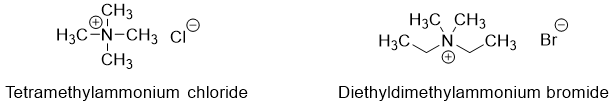
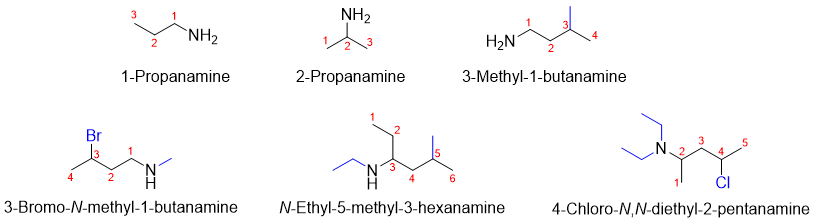
Systematic naming of Amines:
In systematic naming of amines, the “e” at the end of alkyl name is replaced by suffix “amine”. A number is placed in start of the name to designate the carbon atom at which the amine group is attached. Incase if two or more alkyl groups are present in an amine the letter “N” in italic is used to designate the alkyl group attached to nitrogen atom.
Quaternary ammonium salts are systematically names by naming all four alkyl groups is alphabetical order followed by word “ammonium” without using space and then putting the name of the anion.

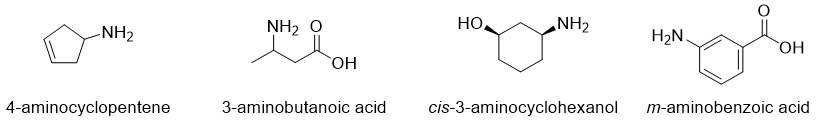
Naming complicated Amines:
Primary amines with complicated structures are named by mentioning -NH2 group by amino. The amino group is treated as a separate substituent with specific numbering to identify its position. For example,
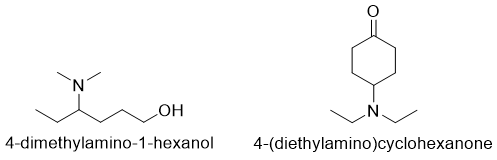
Secondary and tertiary amines are named by treating alkylamino group as a common moiety. The largest or the most complicated alkyl group is taken as a parent chain. For example,
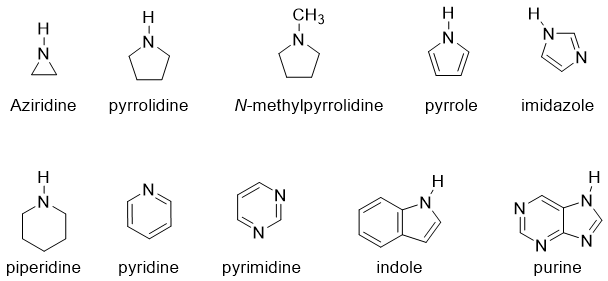
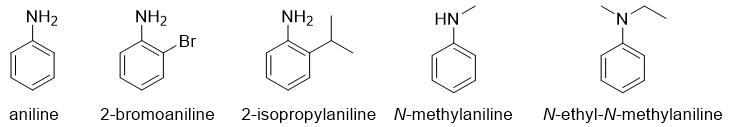
Naming Heterocyclic and Aromatic Amines:
Heterocyclic and aromatic amines are generally known by their historical names. For example,
Following are some examples of common names of nitrogen containing heterocycles.