Synthesis - Azide Reduction
Synthesis of Amines - Azide Reduction
Amines are generally synthesized by reacting ammonia with alkyl halides or tosylates. This method has a drawback of over alkylation. To overcome this problem the alkyl halide or tosylates (primary or secondary) are reacted with azide anion (N3-). A nucleophilic substitution reaction (SN2) takes place and results in the formation of an alkyl azide. Alkyl azide cannot be alkylated further. The alkyl azide upon reduction with lithium aluminum hydride (LiAlH4), H2 in the presence of Palladium catalyst, or triphenylphosphine (Ph3P) in water forms primary amines.

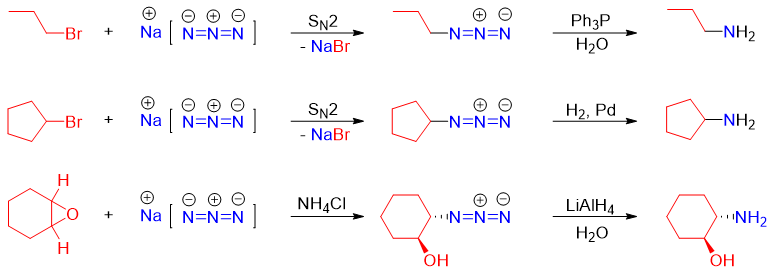
Following are some examples of this reaction.
Mechanism:
The first step involves SN2 reaction in which the azide ion attacks on carbon to which the leaving group is attached. The leaving group leaves and forms alkyl azide.
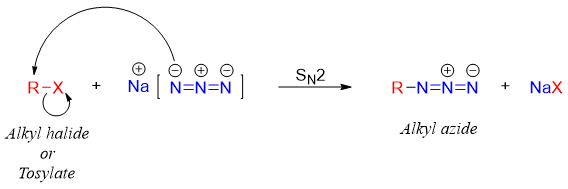
Once the alkyl azide is formed it is carefully reduced to primary amines because the lower molecular weight alkyl azides are explosive in nature thus, the reaction is carefully handled. The alkyl azide is reduced to primary amines by using different reagents.
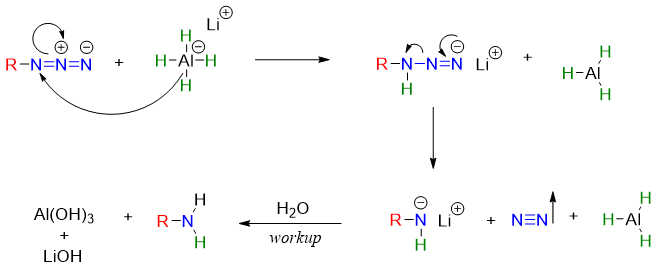
Mechanism of reduction of alkyl azide using LiAlH4:
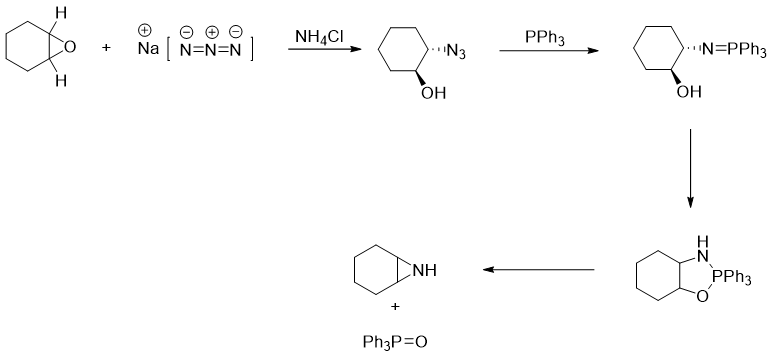
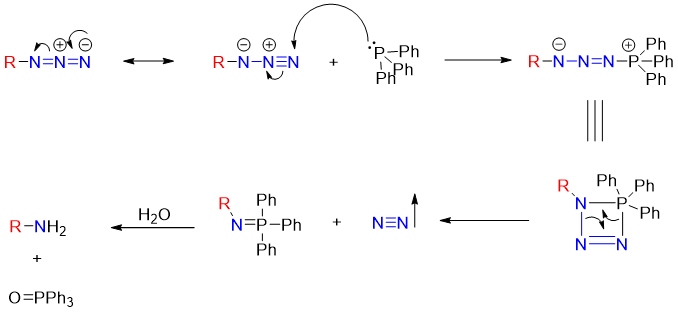
Mechanism of reduction of alkyl azide using Ph3P:
The reaction of alkyl azide with triphenyl phosphene in the presence of water produces primary amines. This reaction is also known as Staudinger reaction. The mechanism of this reaction is given below.
The choice of using different reducing reagents is very important. For example, in following reaction H2 in Pd cannot be used to reduce the azide group because it will also reduce the alkene functionality. Thus, the best choice is either using PPh3 or LiAlH4 reagents.
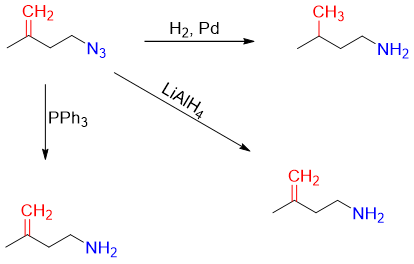
Similarly, in following reaction PPh3 can also produce unwanted products therefore, alkyl azides of such compositions are reduced with LiAlH4 or H2.