Azo Coupling
Azo Coupling
Arenediazonium ions are capable of acting as a weak electrophile in aromatic electrophilic substitution reactions. Arenediazonium ions can react with highly activated benzene derivatives like phenols, anilines, and N-alkylanilines etc. The product formed is called azo compound and the reaction is called diazo coupling.
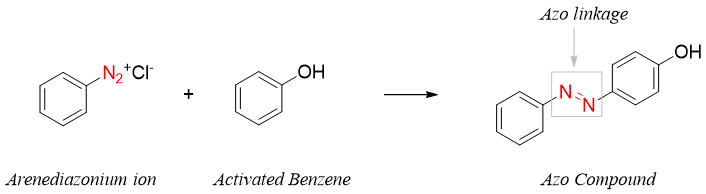
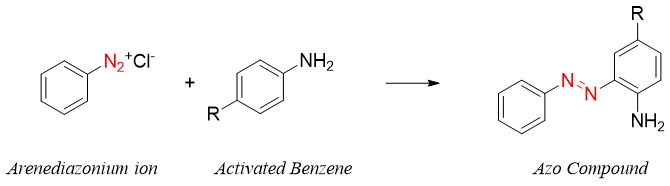
The reaction usually takes place at para position due to large size of the electrophile. If incase the para position is occupied, then the reaction will take place at ortho position.
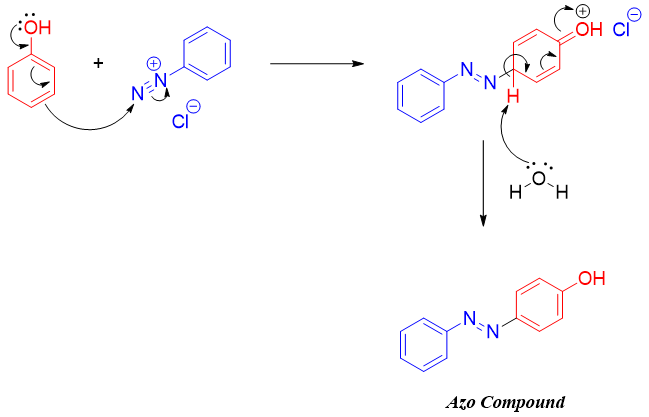
Diazonium coupling reactions follow aromatic electrophilic substitution reactions in which the positively charged -N2+ acts as electrophile.
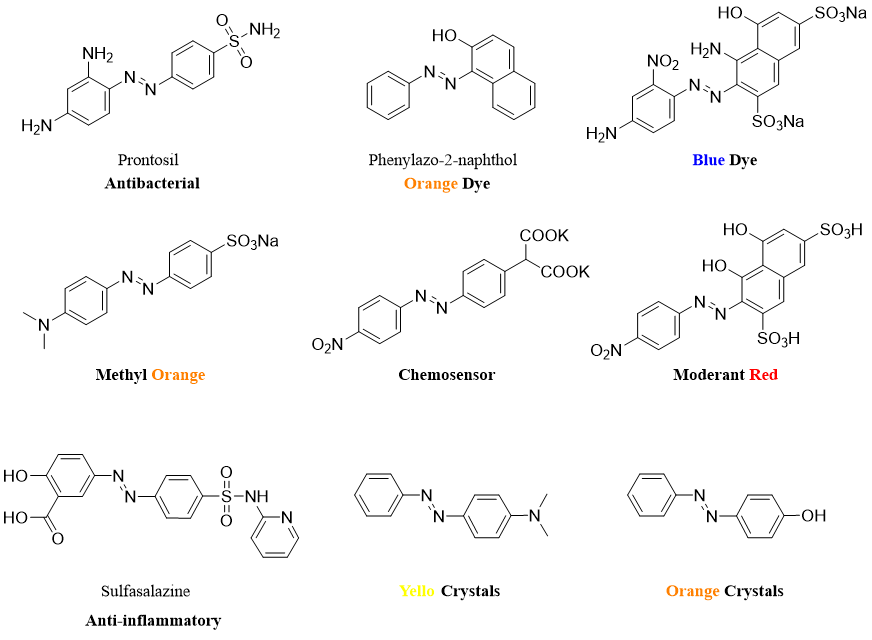
Azo compounds are widely used as dyes to impart colors to leathers, paints, textiles, cosmetics, pharmaceutical products, and foods. The color of azo compounds is due to its extended conjugated pi electron system which absorbs light in visible region of the electromagnetic spectrum.
Following are some examples of azo compounds with their applications.