Acylation of Amines
Acylation of Amines
Primary and secondary amines react with acid chlorides or acid anhydride via nucleophilic acylation reaction to produce N-substituted amides. In this reaction the leaving group of carbonyl compound is replaced by nitrogen atom of attacking amines. The nucleophilic acylation reaction is not given by tertiary amines due to the absence of hydrogen atom on nitrogen atom.
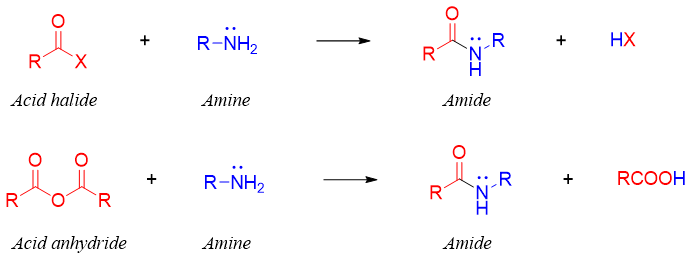
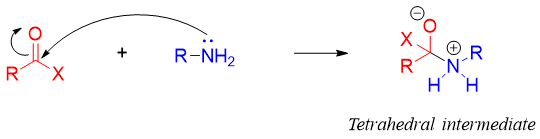
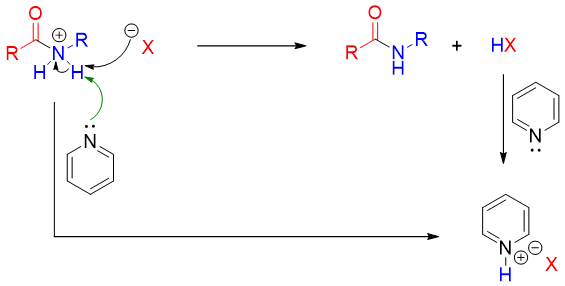
The mechanism involves the attack of amine nitrogen on carbonyl carbon to generate tetrahedral intermediate. Acid chloride and acid anhydrides are the most reactive carbonyl groups.
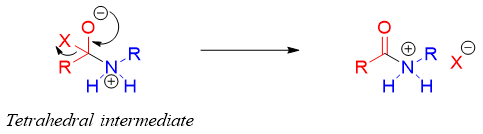
The tetrahedral intermediate formed expels the leaving group to regenerate the carbonyl functionality.
Bases like pyridine and NaOH are used to neutralize the acids formed during the reaction.
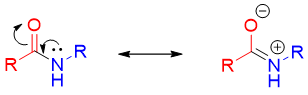
Once the amide is formed in this reaction it does not undergo another acylation reaction. This is due to the stability of the amide due to resonance. The lone pair electrons of amide resonate over carbonyl group bringing a positive charge on nitrogen and making it less nucleophilic and basic.
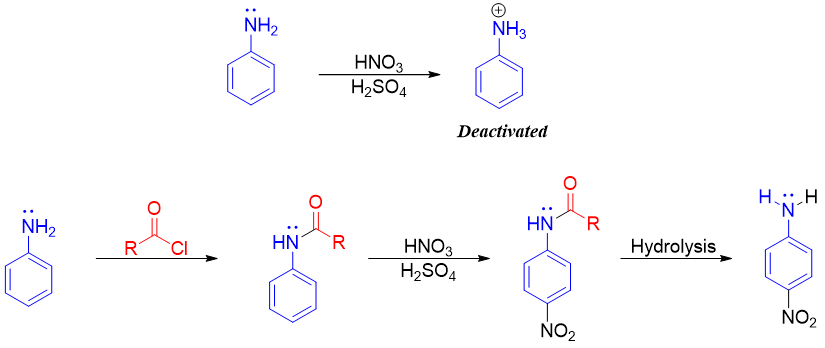
One major synthetic application of amine acylation is the acylation of aniline to acetanilide. The nitration of aniline deactivates the aniline by converting it to anilinium ion. The -NH3+ is strongly deactivating group. Thus, to overcome this problem the aniline is first converted to acetanilide then nitration of acetanilide is performed. At last, the acetanilide is converted back to aniline by acidic or basic hydrolysis.