Enamine Hydrolysis
Enamine Hydrolysis
Enamines are formed by the condensation of an aldehyde or ketone with secondary amines. Enamines are derivatives of enol in which the -OH group is replaced by -NR2.
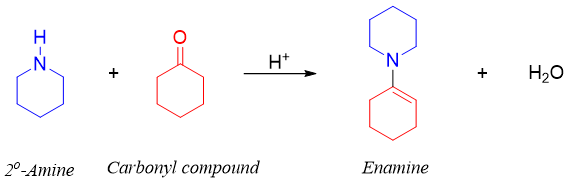
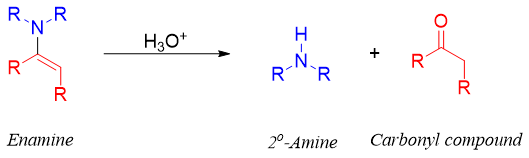
Like imines, in acidic conditions enamines upon hydrolysis produces corresponding carbonyl compound and secondary amines. This reaction is reversible. The secondary amine formed reacts with the acid present in reaction mixture. This stops amine from reacting back with carbonyl compound making enamine again.
The mechanism of enamine hydrolysis starts with the protonation of the double bond. This increases the electrophilic character of iminium carbon thus, making it reactive towards incoming water nucleophile.
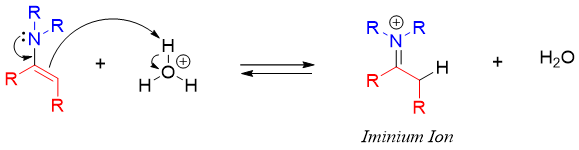
The water molecule adds to the iminium carbon atom followed by the transfer of hydrogen from oxygen atom to nitrogen atom.
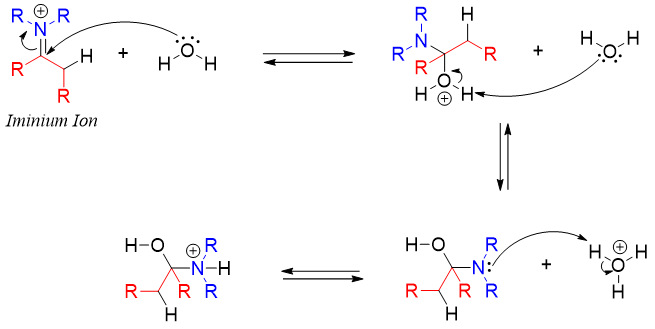
Finally, the lone pair of hydroxyl oxygen moves to carbon atom leaving the neutral amine molecule. The amine further reacts with acid to form ammonium ion. This diminishes the nucleophilic character of amine thus; the reverse reaction is stopped from regenerating enamine again.
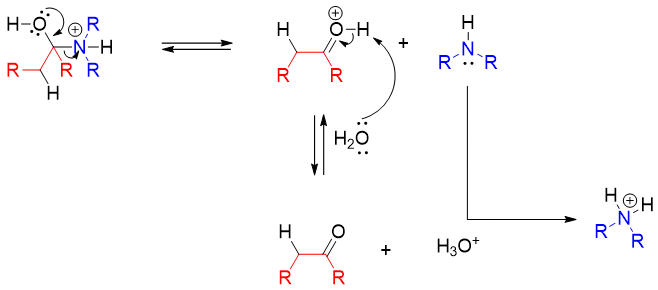
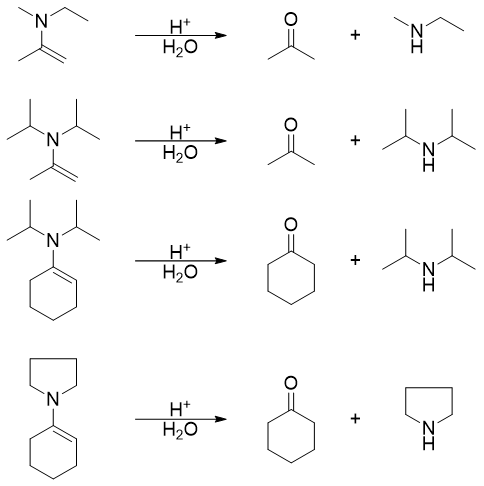
To summarize, enamines upon hydrolysis yields corresponding aldehydes or ketones and secondary amines. The shortcut way to identify the resulting products is to draw an imaginary line between carbon atom and nitrogen atom. The unsaturated bond is made saturated by adding hydrogen atom and the carbon to which nitrogen atom is attached is made carbonyl by adding double bond and oxygen. The vacancy of nitrogen atom is fulfilled by adding hydrogen atom to it. For example,
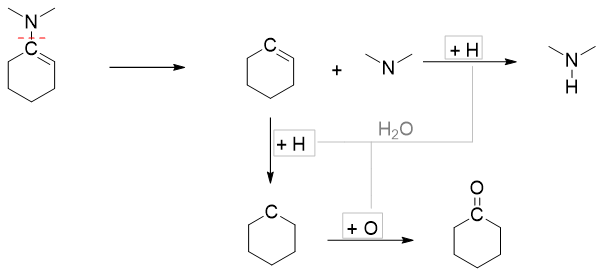
Following are some specific examples of enamine hydrolysis.