Hofmann Elimination
Hofmann Elimination
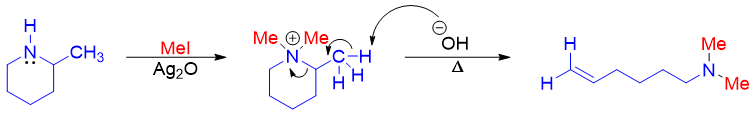
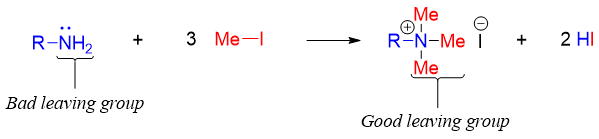
Like alcohols and alkyl halides amines can also be converted into alkenes via E2 elimination reaction. Amines cannot be converted to alkenes directly as the amide (-NH2 or -NHR) groups are not good leaving groups thus, the amino groups are first converted to their corresponding ammonium cations by exhaustive alkylation reaction to make them good leaving groups. The exhaustive alkylation reaction is often done by reacting amines with methyl iodide.
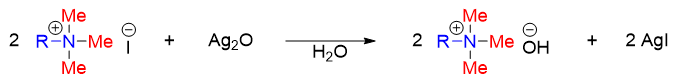
Quaternary ammonium iodide when reacted with base produces alkene via E2 mechanism. The base is provided by converting quaternary ammonium iodide salt to quaternary ammonium hydroxide salt by reacting it with silver oxide (Ag2O).

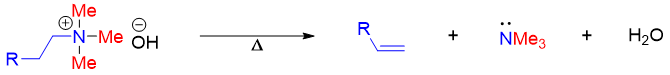
The quaternary ammonium hydroxide salt upon heating produces alkene via concerted E2 elimination.
The conversion of amines to ammonium iodide salt followed by its conversion to ammonium hydroxide salt and the conversion of ammonium hydroxide salt to alkene by heating is collectively called Hofmann elimination reaction.
Unlike alkyl halides the major product in Hofmann elimination reaction follows Hofmann’s rule instead of Zaitsev’s rule. For example,
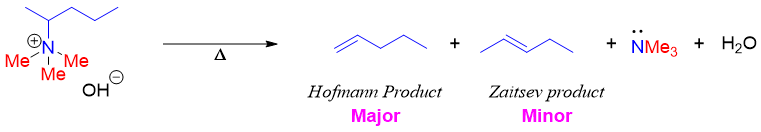
Alkyl halides give Zaitsev product because the more substituted alkenes are more stable thus driving force behind Zaitsev products are stability of substituted alkenes. While in Hofmann elimination reaction the major product is the least substituted alkene. Following reaction shows an example of Zaitsev elimination reaction.
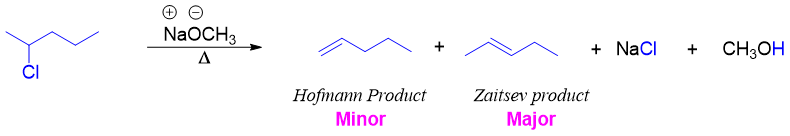
The reason for producing least substituted alkenes by ammonium hydroxide salt is the presence of bulky leaving group [-N(CH3)3+]. As the E2 elimination reaction involves anti-coplanar assembly of the leaving group and the proton. The exceptionally bulky trimethylamine leaving group frequently interferes with anti-coplanar arrangement in Hofmann elimination reaction.
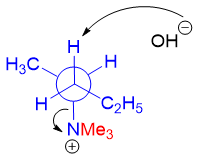
Following figure shows the Newman projection of 2-butanamine. To undergo Zaitsev elimination reaction (between C2 and C3), it should adopt a gauche conformation to obtain anti-coplanar arrangement between H-atom and the leaving group. As shown below, unfavorable gauche interactions take place between the two bulky groups i.e. -N(CH3)3+ and -C2H5. This makes the molecule highly unstable thus, this conformation is not attained
To minimize the interactions between the two bulky groups following conformation must be attained.
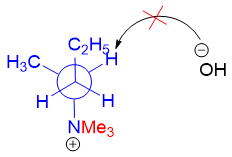
This new conformation is stable, but it cannot undergo E2 elimination reaction because it has lost anti-coplanar arrangement of the hydrogen atom and the leaving group. This shows to produce Zaitsev product the alkylammonium salt cannot maintain the anti-planar geometry due to steric repulsion between the bulky groups.
For the above same molecule to undergo Hofmann elimination reaction (Between C1 and C2), following Newman projection is attained by the 2-butanamine.
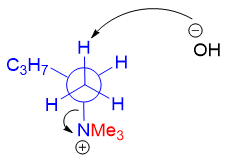
As shown above any of the three protons at C1 can be abstracted by the base as all three protons can form anti-coplanar arrangement with gauche conformation.
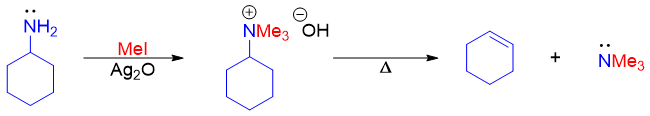
The Hofmann elimination reaction has many applications including determination of structures of complex amines. The complex amines are converted to simpler amines by mean of this reaction. For example, cyclohexanamine upon Hofmann elimination reaction produces cyclohexene.
On the other hand, 2-methylpiperidine upon Hofmann elimination produces N,N-dimethylhex-5-en-1-amine as a major product.