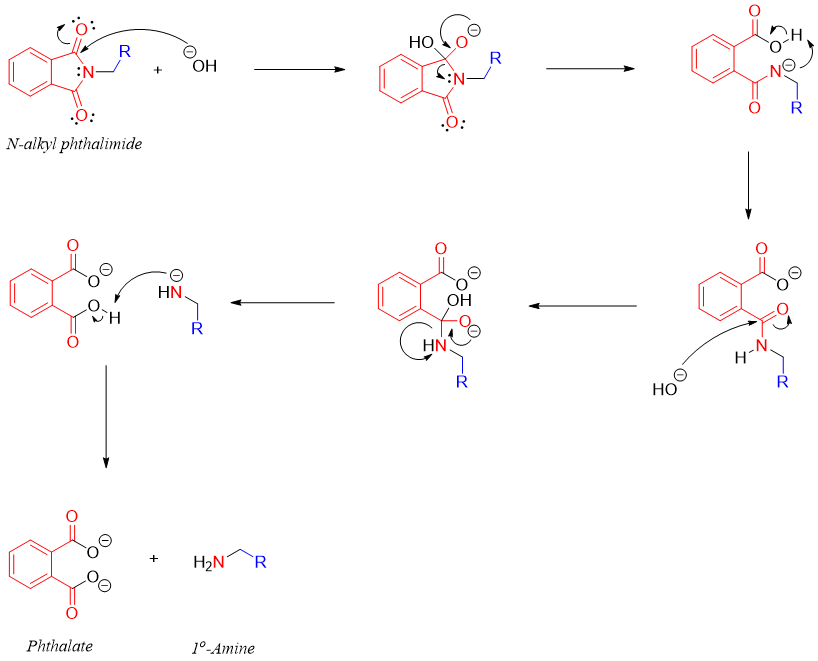
Synthesis - Gabriel Synthesis
Synthesis - Gabriel Synthesis
Gabriel synthesis involves the conversion of alkyl halides to primary amines involving the hydrolysis of an imide.
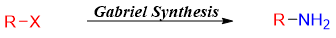
The yields of primary amines synthesized from alkyl halides by the reaction of ammonia through SN2 reaction are poor due to the formation of unwanted byproducts as over alkylation take place
.
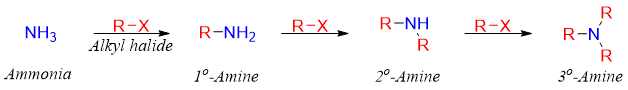
To overcome the problem of over alkylation, primary amines are prepared by means of Gabriel synthesis. This reaction involves the formation of an imide which is hydrolyzed to corresponding primary amines. In Gabriel synthesis phthalimide is deprotonated using base. The resulting phthalimide anion reacts with alkyl halide to produce N-alkyl phthalimide. The N-alkyl phthalimide upon heating with hydrazine produces phthalimide hydrazide and primary amine.
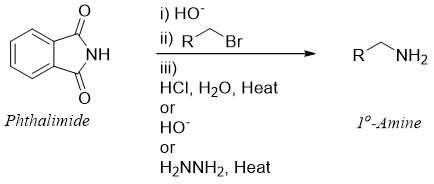
Mechanism:
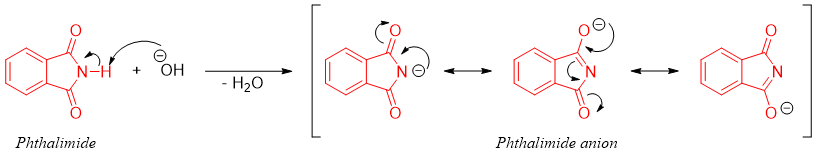
The N-H bond in phthalimide is acidic (pKa = 8.3) which is abstracted by the base and forms phthalimide anion.
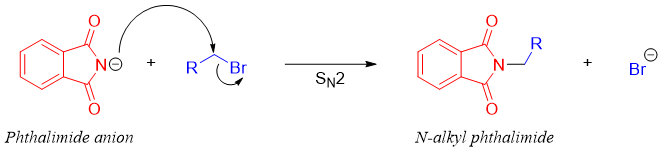
The phthalimide acts as a good nucleophile and can add to alkyl halides or tosylates (usually primary) to undergo SN2 reaction.
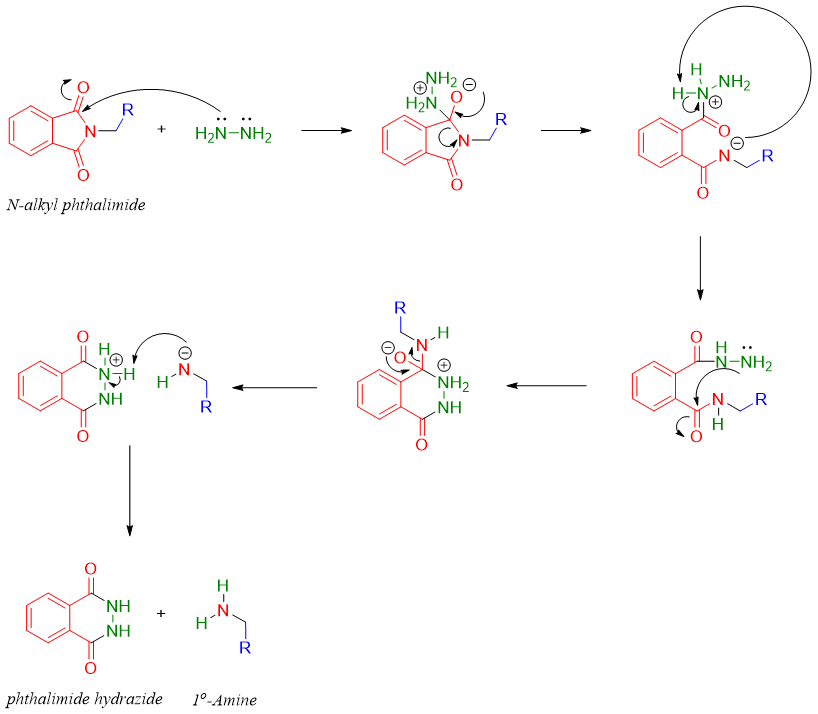
In the final step the N-alkyl phthalimide is heated with hydrazine to produce corresponding primary amines.
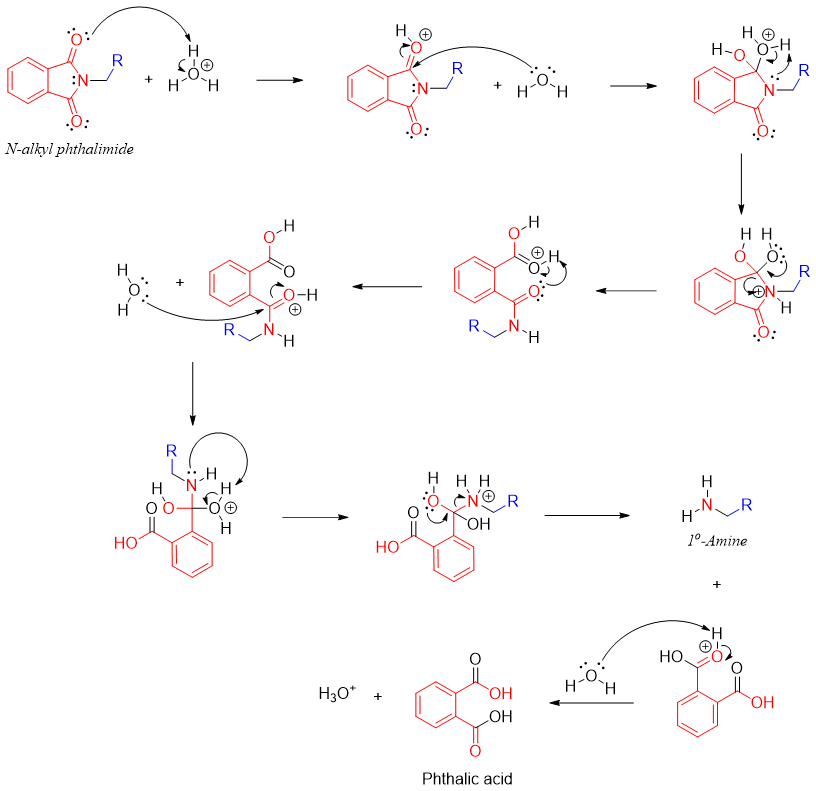
N-alkyl phthalimide can also be hydrolyzed to primary amines and phtalic acid (acid catalyzed) or phthalate (base catalyzed).
The mechanism of acid catalyzed hydrolysis of N-alkyl phthalimide is given below.
The mechanism of base catalyzed hydrolysis of N-alkyl phthalimide is given below.