Formation of Diazonium Salts
Formation of Diazonium Salts
Primary amines when reacted with nitrous acid forms diazonium salt. Nitrous acid being very unstable is generated in situ by the reaction of sodium nitrite (NaNO2) and an acid. The process of conversion of primary amines to diazonium salts is called as diazotization.
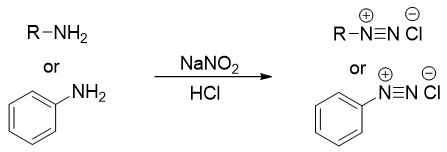
The arenediazonium salts are stable than the alkanediazonium salts. Alkanediazonium salts being very reactive cannot be isolated. Decomposition of arenediazonium salt to phenol takes place at room temperature. To overcome this problem, the diazotization reaction is carried out at low temperatures. At low temperatures the formation of phenol is suppressed.
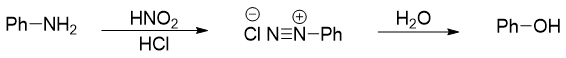
The mechanism of formation of diazonium salt involves the formation of nitrosonium ion. Nitrosonium ion is synthesized from nitrous acid (HNO2). Nitrous acid being unstable is commonly generated in situ by treating sodium nitrite (NaNO2) with dilute acid.
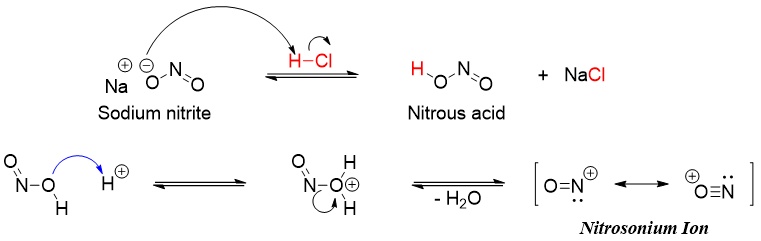
Nitrosonium ion readily reacts with primary amines to produce corresponding diazonium salt.
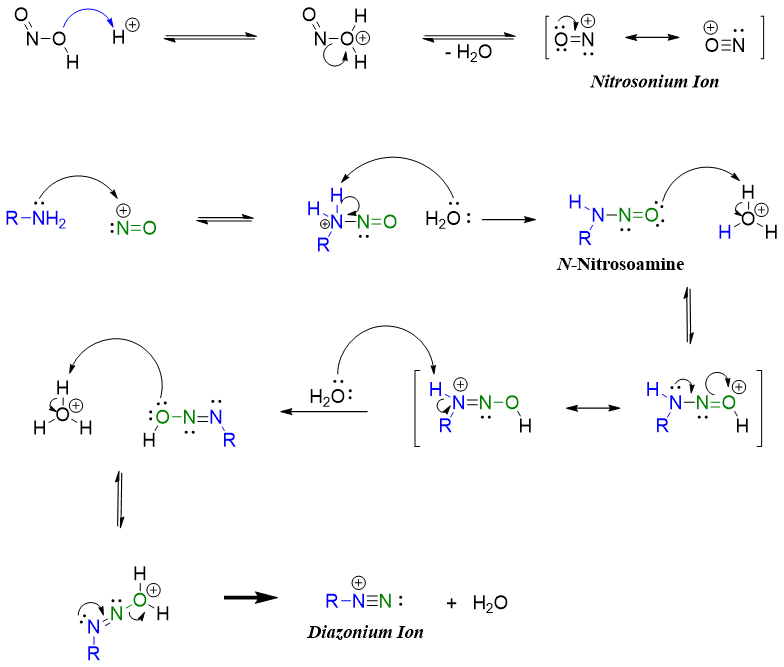
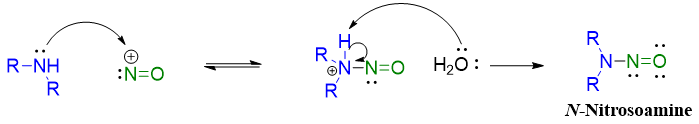
The reaction of secondary amines with nitrous acid stops at N-nitrosamine stage. Nitrosoamines of secondary amines are stable due to the absence of N-H proton which tautomerizes in mechanism of primary amines shown above.
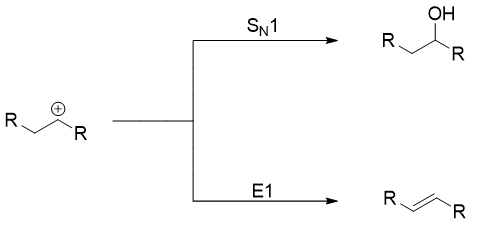
Diazonium salts of aliphatic primary amines are usually not important due to their highly unstable nature both at room and low temperatures. As soon they are formed, they decompose to form corresponding carbocation and nitrogen gas.
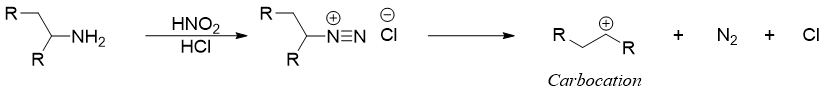
The carbocation formed can undergo either SN1 reaction or E1 elimination reaction to produce alcohols or alkenes respectively.