The Fischer Proof
The Fischer Proof
In 1891, Emil Fischer determined the relative configuration of all stereocenters in (+)-glucose. He selected (+)-glucose due to its natural abundance. Fischer knew that (+)-glucose is an aldohexose and has four chiral centers and there are 16 possible stereoisomers (2n = 24 = 16). At that time there was no direct method to determine the absolute configuration of each chiral carbon atom of (+)-glucose hence, Fischer determined the relative configuration of each stereocenter.
Fischer started by assuming that the naturally occurring glucose had a D-configuration. In D-configuration the hydroxyl group bonded to the stereocenter farthest from the carbonyl group is oriented at the right hand side of the Fischer projection. He then started determination of each orientation of other hydroxyl groups relative to this stereocenter.
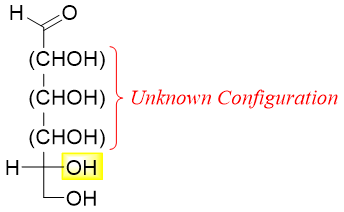
The stereoisomers of aldohexose exist in eight pairs of enantiomers. Out of these eight pairs only one pair is for (+)-glucose. Hence, Fischer only considered one set of these eight pairs. Following structures show the eight possible structures of aldohexose with D-configurations. The remaining eight are their enantiomers.
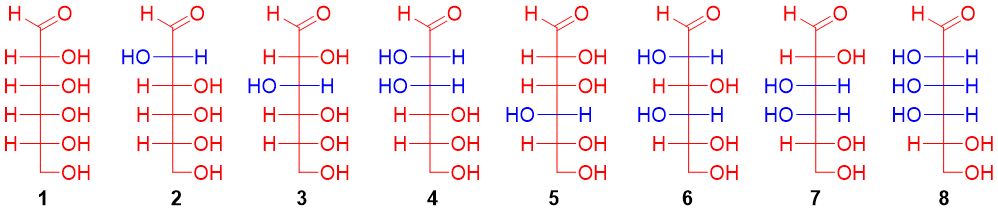
Fischer used the following steps to determine the stereochemistry of (+)-glucose, to determine the configuration of each chiral center of glucose.
Step 1: Kiliani-Fischer synthesis of Arabinose:
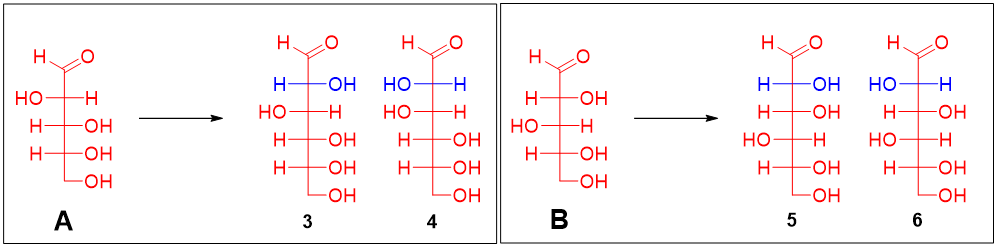
The Kiliani-Fischer synthesis forms two epimers with different configurations at C2 position. Kiliani-Fischer synthesis of arabinose (aldopentose) forms (+)-glucose and (+)-mannose. This suggests that (+)-glucose and (+)-mannose are epimers of each other at C2 position and have the same stereochemistry at remaining chiral carbons. Therefore, the structures of (+)-glucose and (+)-mannose are either 1 and 2, 3 and 4, 5 and 6 or 7 and 8.
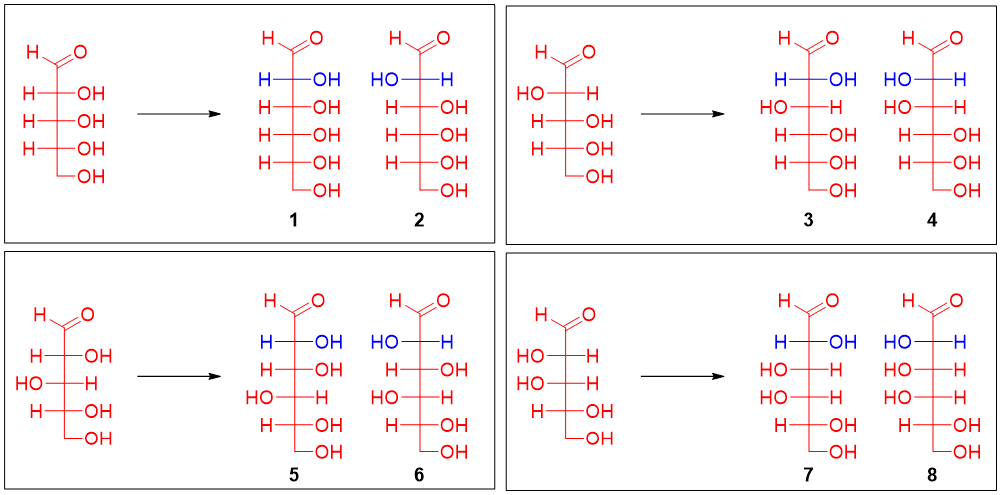
Step 2: Oxidation of Glucose and Mannose to optically active Aldaric acid:
Furthermore, it was found that the glucose and mannose when oxidized formed optically active aldaric acid. This suggests that glucose and mannose do not have any plane of symmetry. The aldaric acids formed by the oxidation of all four pairs is depicted below.
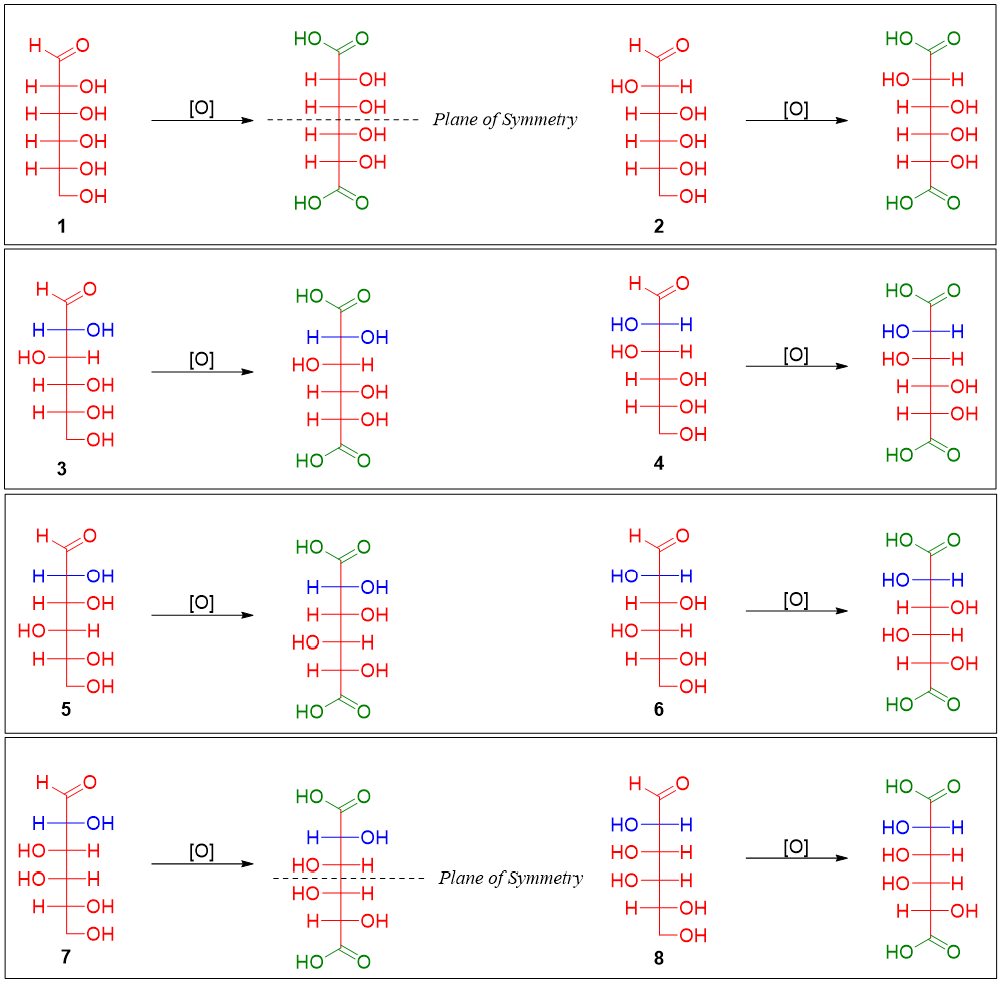
The pairs in which one of the members formed an aldaric acid with a plane of symmetry can be eliminated. Thus, sugar 1 (and its epimer 2) and sugar 7 (and its epimer 8) are eliminated. Now, glucose and mannose are either pairs 3 and 4 or pairs 5 and 6.
Step 3: Oxidation of Arabinose to optically active Aldaric acid:
From step 2 it can be concluded that the structure of arabinose (aldopentose) is either A or B as shown below.
Therefore, if arabinose is oxidized to aldaric acid it should not produce optically inactive aldaric acid because we know that the glucose and mannose are optically active. Following figure shows the oxidation of A and B.
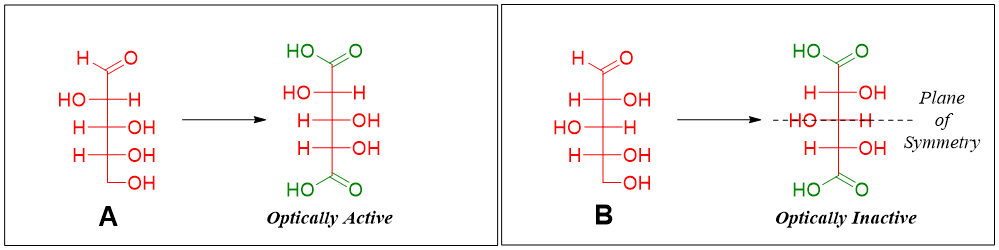
Hence, sugar A is the structure of Arabinose.
Step 4: Interchanging functional groups on present on terminal positions:
At this stage only two structures were left (3 and 4).
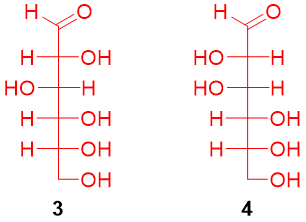
To confirm which structure belongs to glucose the terminal functional groups (-CHO and -CH2-OH) present at the terminal positions are interchanged. After that, the resulting structures are rotated at 180°.
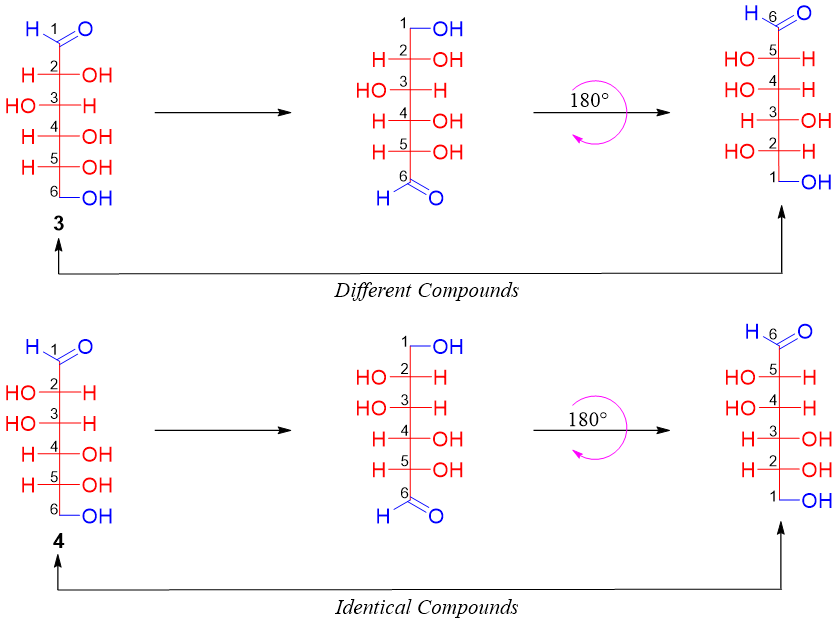
As shown above compound 3 gives different compound while compound 4 gives identical compound.
The glucose molecule gives different molecules when its terminal groups are interchanged whereas, mannose gives identical compounds when its terminal groups are interchanged. Hence, it is concluded that compound 3 is the (+)-glucose molecule.
“The Proof is Complete”
The Fischer proof was considered remarkable because it was performed at the time when remarkably simple analyses like melting point determination and determination of optical rotations were considered the most advanced techniques available to the scientists. The structure of (+)-glucose was confirmed by X-ray crystallography in 1951. The structure was the same as assumed by Emil Fischer 50 years earlier.
