Mutarotation
Mutarotation
The anomers of a monosaccharides are diastereomers of each other as the configuration at the anomeric carbon atom is different. Due to this the anomers of the same monosaccharide have different physical properties. Following figure shows the anomers of D-glucopyranose and their physical properties.
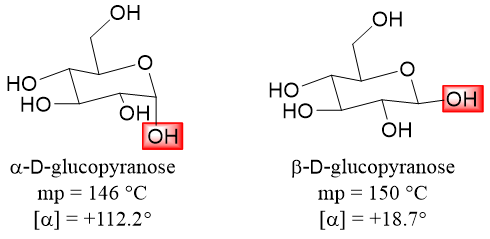
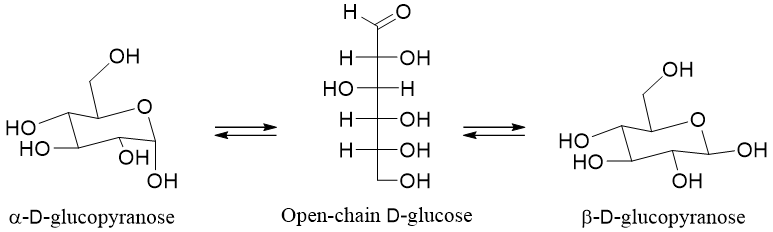
When D-glucose is dissolved in water it forms an equilibrium mixture of three different forms i.e., a) open-chain form, b) α-D-glucopyranose, and c) β-D-glucopyranose. When the crystallization of D-glucose is done at room temperature it forms pure crystals of α-D-glucopyranose. On the other hand, if crystallization is done above 98 °C it forms pure crystals of β-D-glucopyranose.
When any one of the pure anomers of D-glucose is dissolved in water a change in specific rotation occurs. When β-D-glucopyranose is dissolved in water its specific rotation increases from +18.7° to +52.6°. Whereas, if α-D-glucopyranose is dissolved in water its specific rotation decreases from +112.2° to +52.6°. This change in specific rotation is called mutarotation.
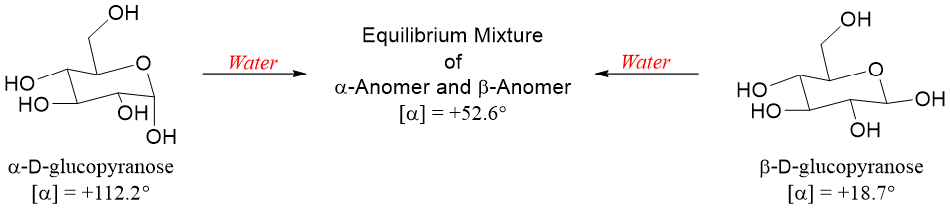
The process of mutarotation occurs because when an anomer is dissolved in water the hemiacetal opens to form an acyclic D-glucose. The acyclic D-glucose then recyclizes to form the α- and β- anomers again. Eventually, the three forms of D-glucose (acyclic open-chain D-glucose, α-D-glucopyranose, and α-D-glucopyranose) reach equilibrium concentrations. The specific rotation of this equilibrium mixture is +52.6°. At equilibrium, the concentration of α-D-glucopyranose is 36% while, concentration of β-D-glucopyranose is 64%. The β-D-glucopyranose is present in high concentrations due to its higher stability as the hydroxyl group at anomeric position in β-D-glucopyranose is at equatorial position.
D-galactopyranose also shows mutarotation when dissolved in water. When any one of the pure anomers of D-galactose is dissolved in water a change in specific rotation occurs. When β-D-galactopyranose is dissolved in water its specific rotation increases from +52.8° to +80.2°. Whereas, if α-D-galactopyranose is dissolved in water its specific rotation decreases from +150.7° to +80.2°.

The percentage of these two anomers can be calculated as.
a (+ 150.7°) + b (+ 52.8°) = + 80.2° ----- (1)
Taking open chain form concentration is negligible.
a + b = 1
Or,
b = 1 – a
Substituting value of b in eq. 1.
a (+ 150.7°) + (1-a) (+ 52.8°) = + 80.2°
Solving for a.
a = 28%
Hence, the concentration of each anomer in an equilibrium mixture is.
