Side Reactions of Monosaccharides in Base
Side Reactions of Monosaccharides in Base
Monosaccharides are multifunctional organic compounds and can undergo different functional group reactions. In solutions monosaccharides exist in open chain acyclic form therefore, they can undergo the usual reactions of alcohols, ketones, and aldehydes. Sugars when treated with base can undergo two types of reactions.
Epimerization:
When sugars are reacted with base the proton present at alpha position (next to carbonyl group) is abstracted resulting in the formation of an enolate.
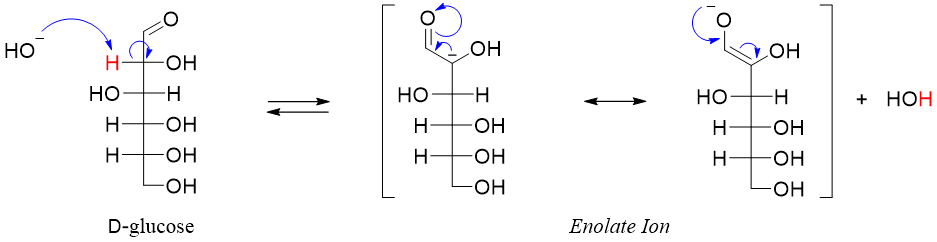
The stereochemistry of C2 in enolate is lost. The resulting enolate can get re-protonated when reacted with water. The addition of proton can take place at either face of the enolate forming two epimers with different configuration at C2.
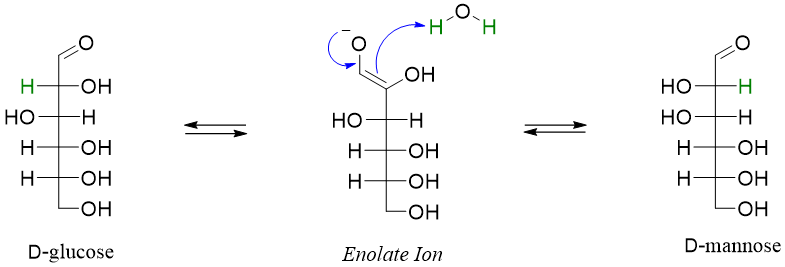
As the reaction is forming a pair of epimers (different configurations at C2 position) therefore it is called as epimerization reaction.
The Enediol Rearrangement:
Sugars when treated with base undergo another base-catalyzed side reaction called the enediol rearrangement. In this rearrangement the carbonyl group is either shifted upward or downwards. Following mechanism shows the conversion of D-glucose into D-fructose (downward movement of carbonyl) when reacted with base.
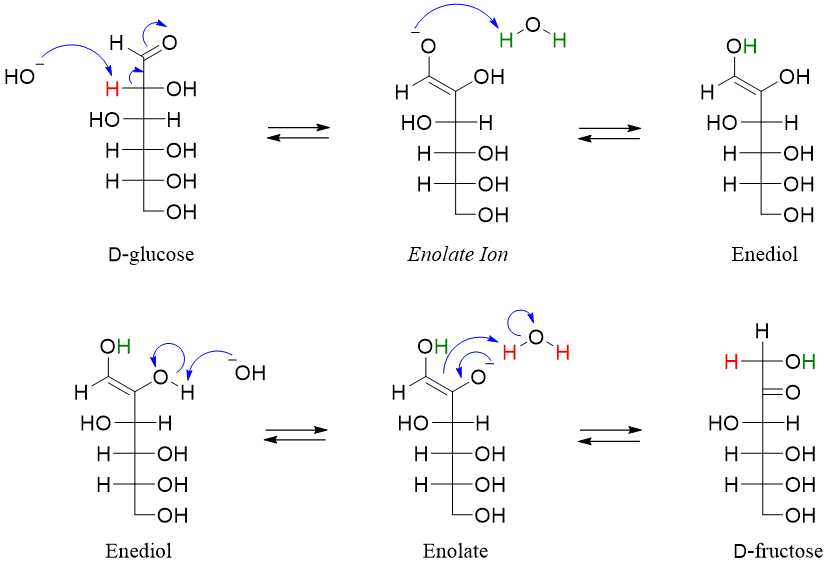
Following enediol rearrangement mechanism shows the movement of carbonyl group downwards when D-fructose is treated with base.
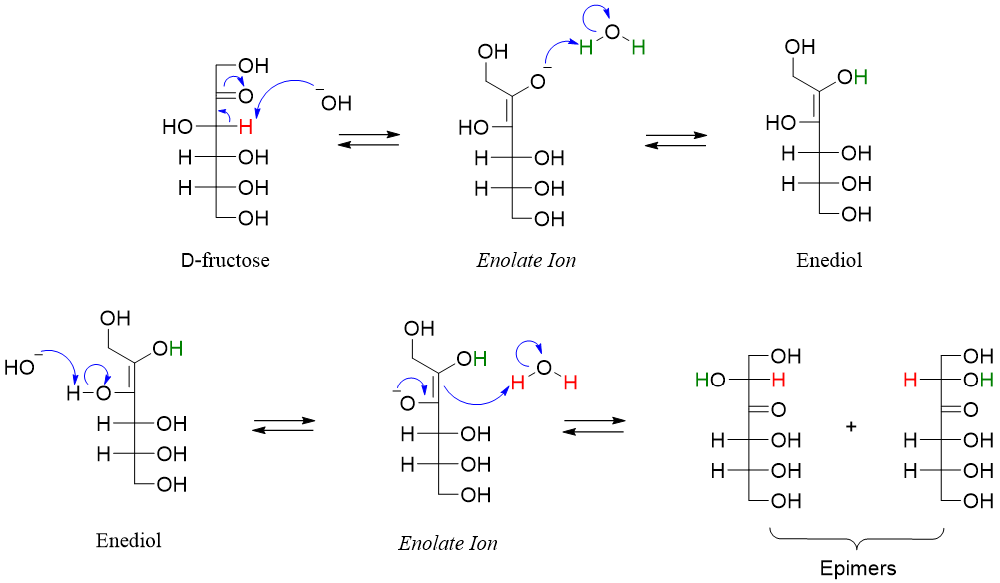
Following enediol rearrangement mechanism shows the movement of carbonyl group upwards when D-fructose is treated with base.
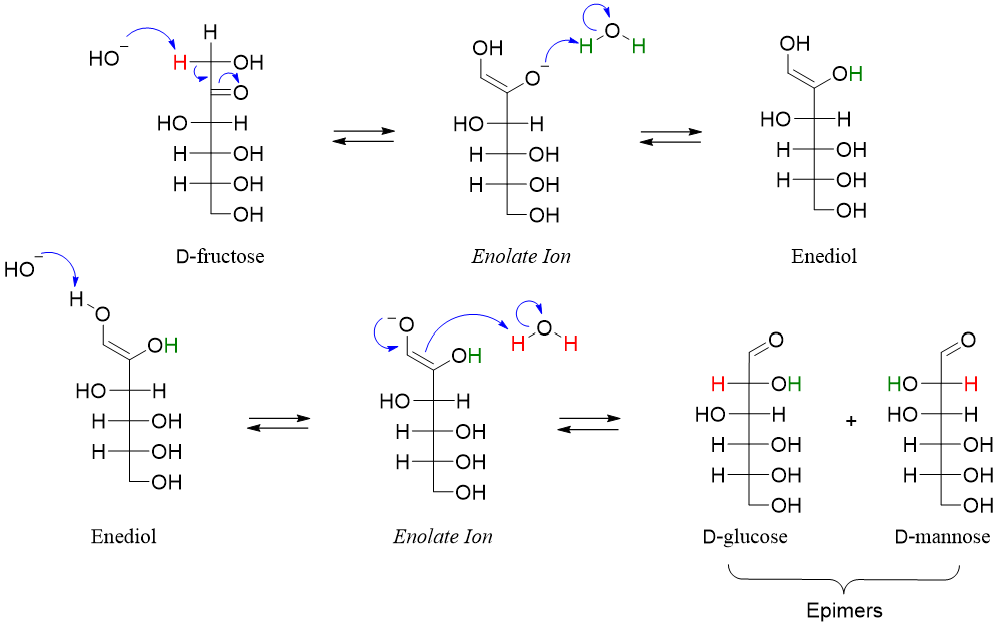
Hence, we can conclude that when aldohexose is treated with base it will result in the formation of mixture products containing epimers and one or two ketoses. Whereas ketohexoses upon reaction with base can form aldohexose (movement of carbonyl group upwards), other ketoses (movement of carbonyl group downwards) and their epimers.
