The Family of D-Ketoses
The Family of D-Ketoses
The family of D-ketoses consists of sugars having ketone functional group at position two and a stereocenter with a hydroxyl group at the right hand side of the Fischer projection present farthest from the carbonyl group.
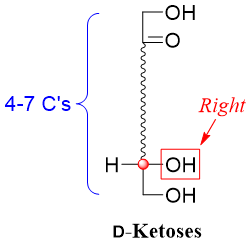
The simplest D-ketose is the D-dihydroxyacetone. Using dihydroxyacetone as a reference one can devise higher D-ketoses by placing carbon atoms bonded to hydrogen atom (-H) and hydroxyl group (-OH) between C2 and C3 of dihydroxyacetone. For example
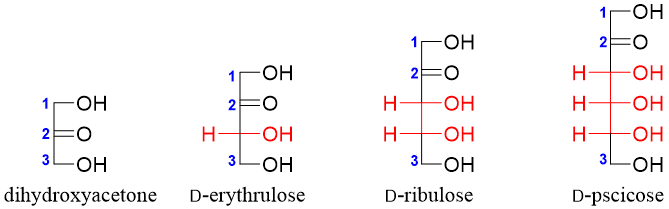
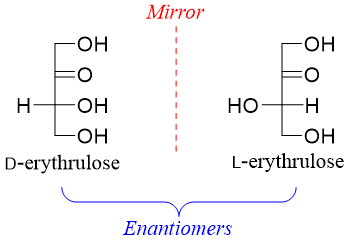
Dihydroxyacetone (ketotriose) has no stereocenter while, D-erythrulose (ketotetrose) has one chiral carbon therefore it has two stereoisomers (2n = 21 = 2). Both stereoisomers are enantiomers of each other.
ketopentoses have two stereocenters hence, they will have four stereoisomers (2n = 22 = 4). Two of the stereoisomers are D-ribulose and D-xylulose and the other two will be their enantiomers (L-ribulose and L-xylulose).
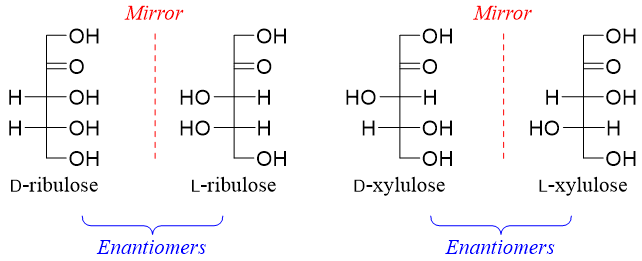
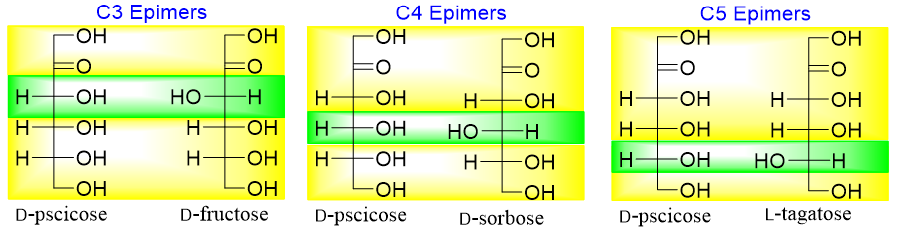
Ketohexoses have three stereocenters hence, they will have eight stereoisomers (2n = 23 = 8). Four of the stereoisomers are D-pscicose, D-fructose, D-sorbose, and D-tagatose and the other four will be their enantiomers (D-pscicose, D-fructose, D-sorbose, and D-tagatose).
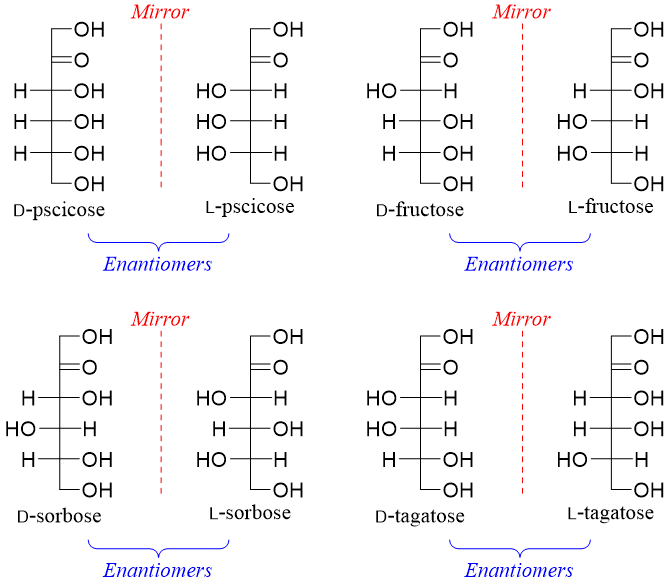
Following figure depicts the epimers of ketohexoses.