Reducing vs Non Reducing Sugars
Reducing and Nonreducing Sugars
To understand the concept of reducing and nonreducing sugars one should be familiar with the terms hemiacetals and acetals. In organic chemistry hemiacetals are those compounds in which a carbon atom is single bonded to hydroxyl group (-OH) and single bonded to alkoxy group (-OR). Whereas, in acetals the carbon atom is single bonded to two alkoxy (-OR) groups.

Reducing Sugars:
Those sugars which are capable of donating its electrons to other molecules by oxidizing them are known as reducing sugars. Hence, reducing sugars can react with oxidizing agents. In this reaction the oxidizing agent is reduced while the reducing agent is oxidized.
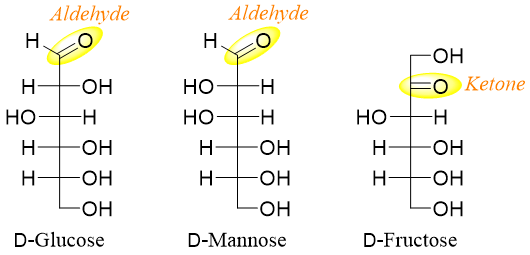
Reducing sugars either contain aldehyde or ketone functional groups or they contain a hemiacetal group. Sugars with hemiacetal group are reducing sugars as they are in equilibrium with the open chain sugars in solution. Following are some examples of reducing sugars containing ketone or aldehyde functional groups.
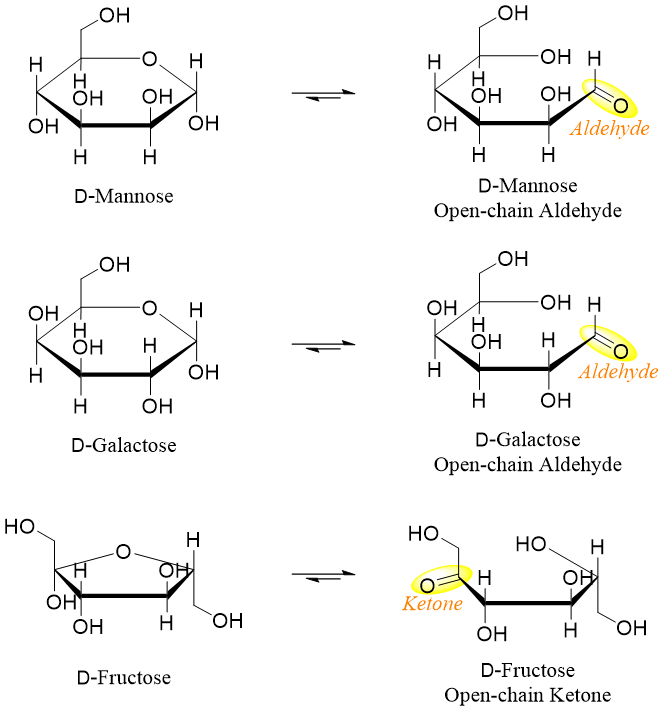
Monosaccharides with cyclic hemiacetal structures are also reducing sugars. These hemiacetals exist as open chain aldehyde or α-hydroxy ketone in solutions. For example,
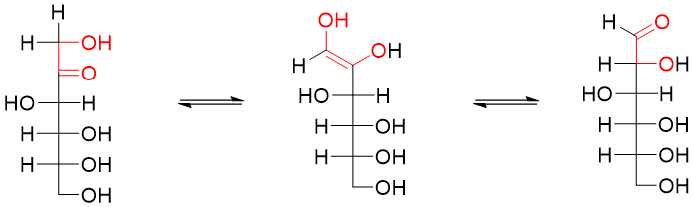
D-fructose containing ketone functional groups are oxidized due to the presence of hydroxyl groups on the adjacent carbon atom. α-hydroxy ketones tautomerize to corresponding aldehydes as shown below.
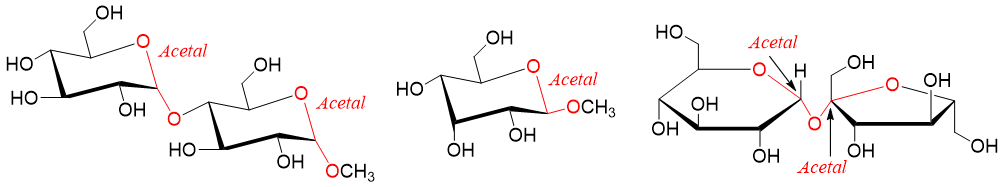
Disaccharides can also function as a reducing sugars. For example,
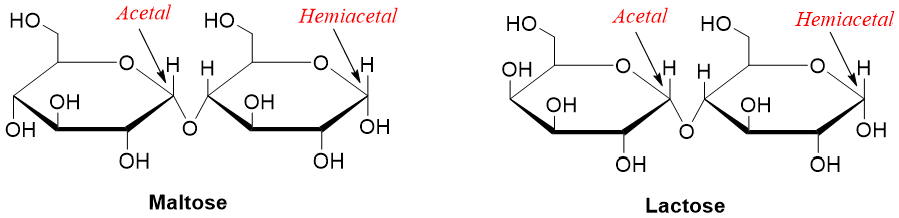
Non-Reducing Sugars:
Those sugars which are incapable of donating its electrons to other molecules by oxidizing them are known as nonreducing sugars. Hence, nonreducing sugars cannot react with oxidizing agents. Nonreducing sugars exist in the form of acetal or ketal forms. This means that nonreducing sugars do not exist in the form of free aldehyde or ketone.
Following are some examples of non reducing sugars.