Ether and Ester Formation
Ether and Ester Formation
Monosaccharides contain two types of functional groups, carbonyl group and hydroxyl group therefore, they have similar chemistry of these two groups. For example, the hydroxyl groups of monosaccharides can be changed into ethers and esters. Due to the presence of a considerable number of hydroxyl groups, monosaccharides are highly soluble in water but insoluble in organic solvents. Furthermore, monosaccharides are difficult to be purified and crystallized as they form a saturated syrup instead of crystals. To overcome this problem, scientists convert monosaccharides to their corresponding ethers and esters derivatives to make them soluble in organic solvents and purify and crystallize them easily.
Ether Formation:
Sugars are converted to corresponding ethers using Williamson ether synthesis method by treating them with alkyl halides in the presence of base. Since a strong base can degrade sensitive sugars therefore silver oxide (mild base) is used as an alternative.
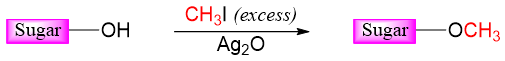
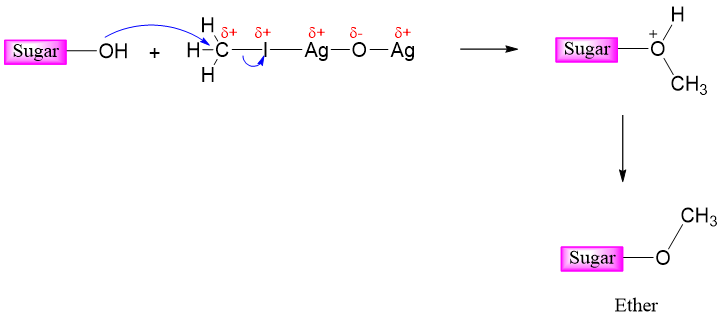
Silver oxide is used to increase the electrophilicity of alkyl halide carbon as it polarizes the bond between carbon atom and iodine atom.
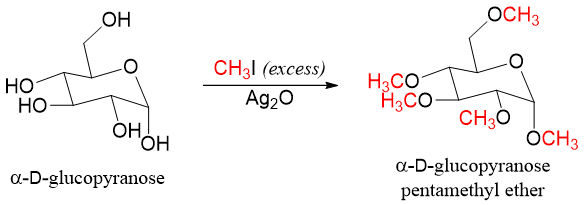
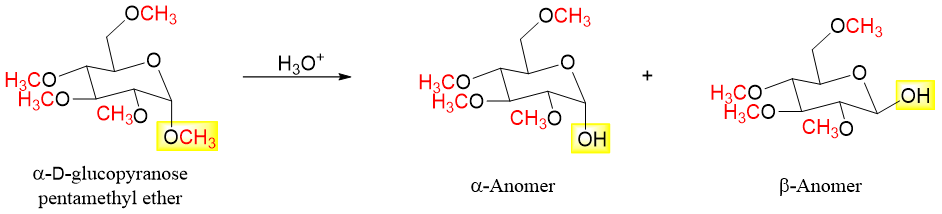
When monosaccharide is reacted with excess methyl iodide in the presence of silver oxide all hydroxyl groups are converted into ethers. For example,
When alkylated sugar is reacted with aqueous acid the acetal ether is hydrolyzed whereas, the remaining four ether groups are intact and are not hydrolyzed. The hydrolysis of single glycoside yields both anomers.
Ester Formation:
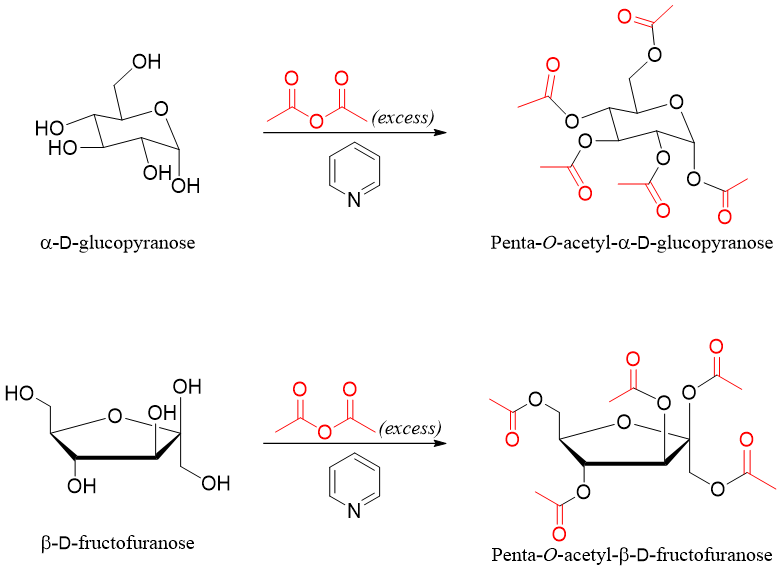
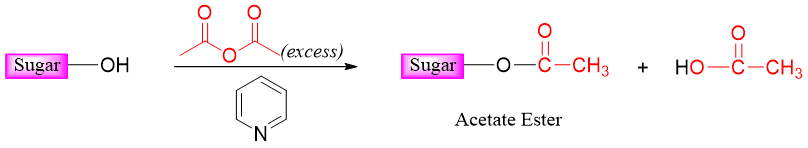
Esterification of monosaccharides is conducted by treating the sugar with acetyl chloride or acetic anhydride in the presence of a mild catalyst base (pyridine). All hydroxyl groups of sugar react and are converted into ester groups.
Mechanism:
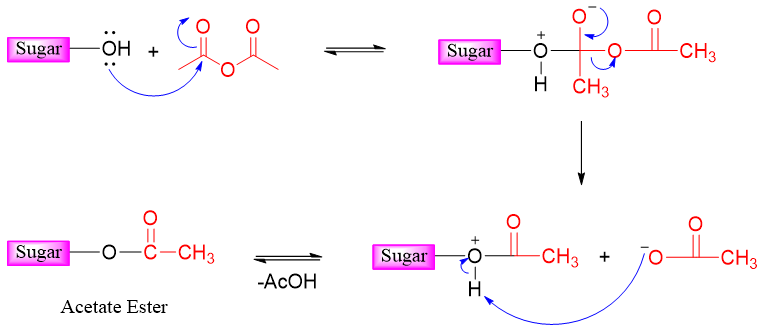
In esterification reaction the stereochemistry of anomeric carbon is preserved. Following examples show the esterification reaction of different sugars.