Glycosides
Formation of Glycosides
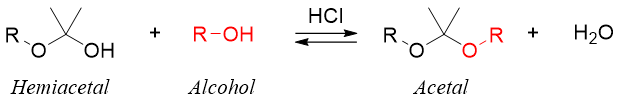
The reaction of monosaccharides with alcohols in acidic medium results in the conversion of hemiacetals into an acetal called glycoside. This is the method for converting reducing sugars into nonreducing sugars. The general reaction is shown below.
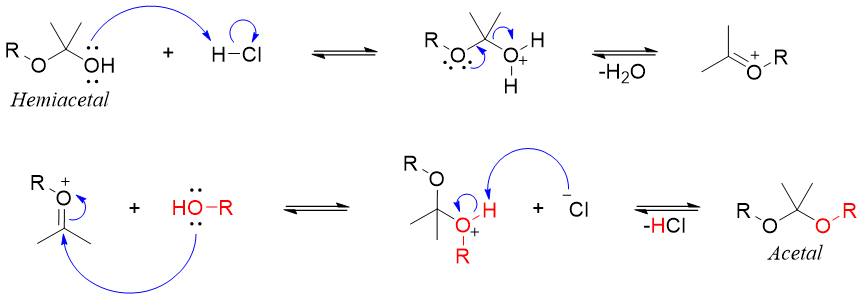
The mechanism for this reaction is as follows.
The bond formed between the anomeric carbon and alkoxide group is called glycosidic bond. The glycosides of six-membered rings are called pyranosides. For example, glycoside of glucose is called glucopyranoside. Similarly, glycoside of five-membered ring sugars is called furanoside. For example, glycoside of ribose sugar is called ribofuranoside. The name ending with suffix -oside stands for nonreducing sugars while, the name ending with suffix -ose stands for reducing sugars.
Following example shows the formation of glucosides of α-D-glucose.
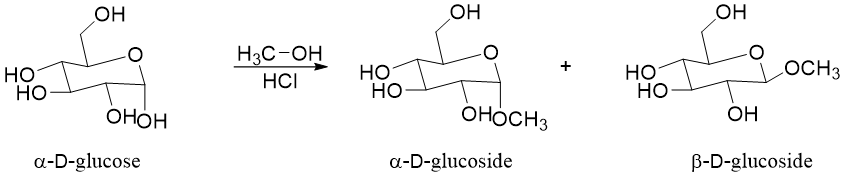
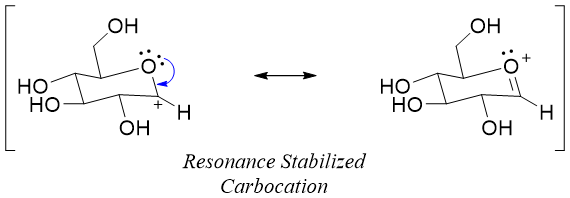
As shown above the single anomer of glucose is forming two glycosides. This is due to the formation of the planar carbocation formed during the reaction mechanism.
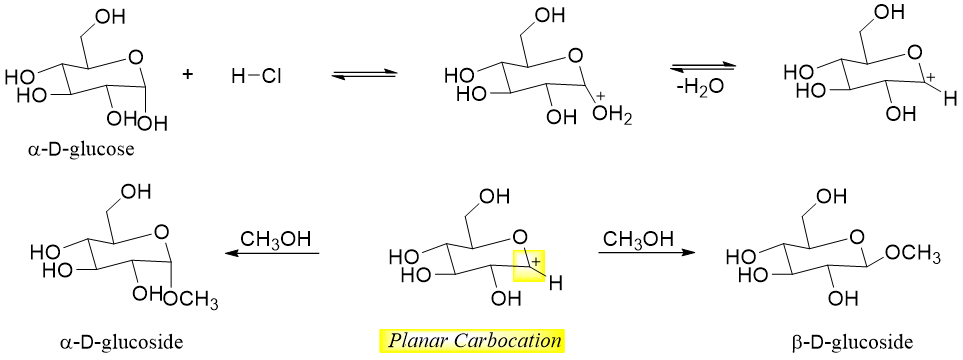
This reaction only takes place at the hemiacetal group (anomeric carbon). This is due to the formation of resonance stabilized carbocation during the reaction.
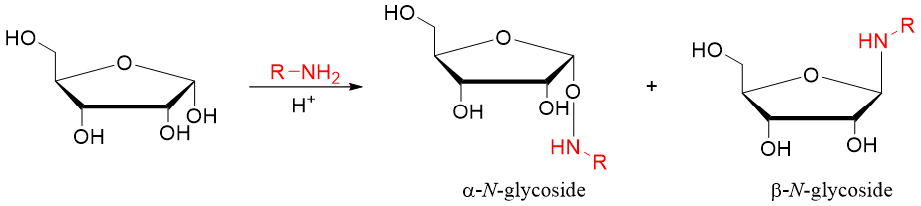
If the reducing sugar is reacted with amines instead of alcohols the resulting product is called N-glycoside. The glycosidic bond is formed between the anomeric carbon and nitrogen atom.
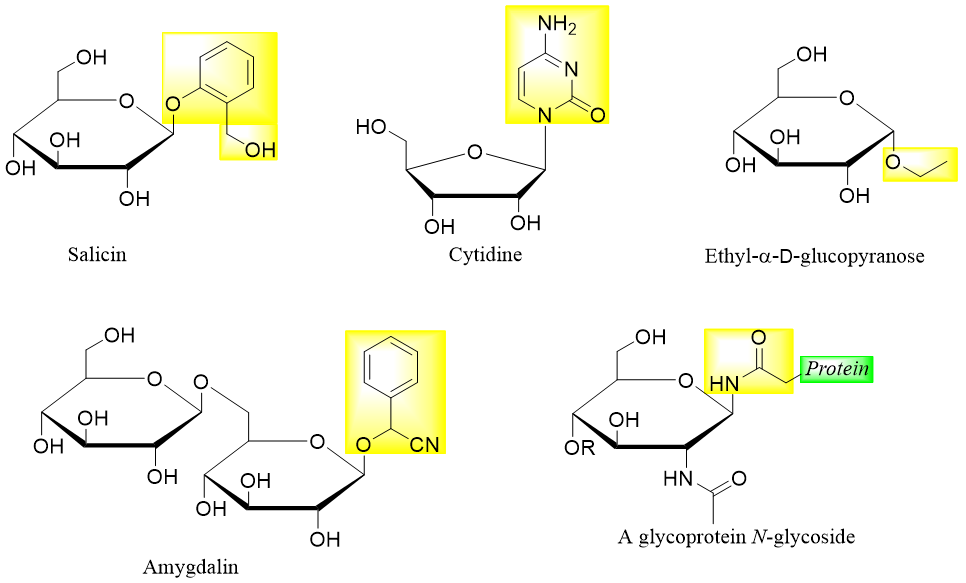
Following are some examples of different glycosides formed by reaction of different aglycons with reducing sugars.