Monosaccharides
Monosaccharides
Monosaccharides are the simplest carbohydrates. They are also termed as simple sugars. Monosaccharides contain three to seven carbon atoms with a carbonyl group present at either carbon-1 (aldoses) or carbon-2 (ketoses). Monosaccharides are drawn vertically with the carbonyl group present at the top and the hydroxyl groups on the remaining carbon atoms.
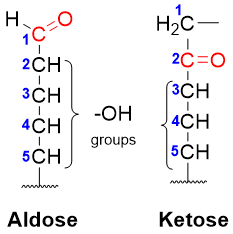
Following figure depicts a few examples of simplest monosaccharides.
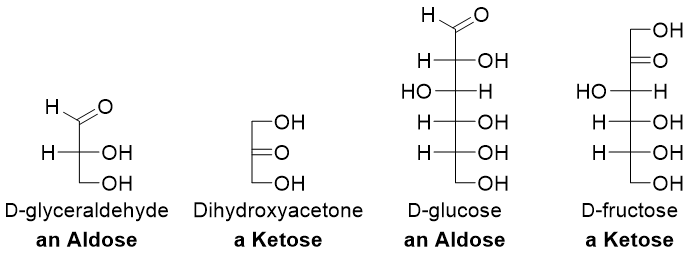
Both D-glyceraldehyde and dihydroxyacetone have the same molecular formula (C3H6O3) hence they are constitutional isomers of each other. Similarly, D-glucose and D-fructose are constitutional isomers of each other. A monosaccharide is called triose if it contains three carbon atoms, tetrose if it contains four carbon atoms, pentose if it contains five carbons, hexose if it contains six carbon atoms and heptose if it contains seven carbon atoms.
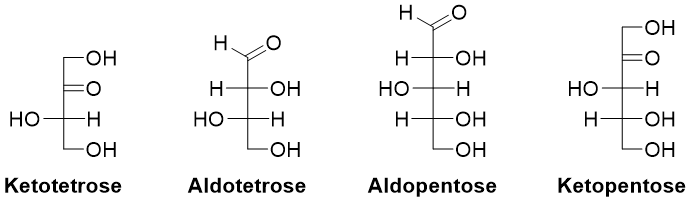
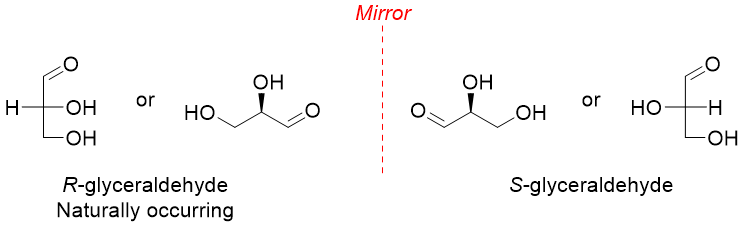
Except dihydroxyacetone all carbohydrates contain at least one stereocenter. Each chiral monosaccharide has two enantiomers. The stereoisomer with R configuration exists naturally.
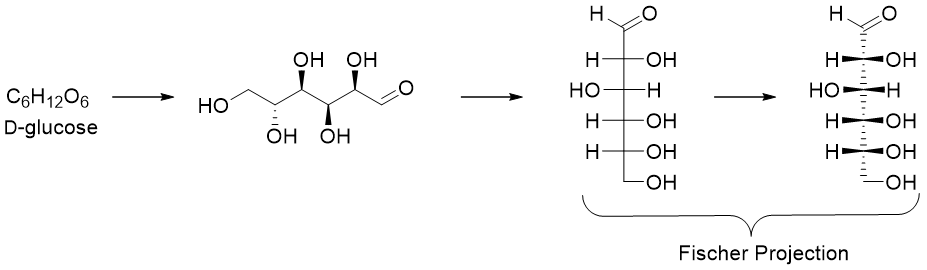
The structures of carbohydrates are drawn by a cross formula called the Fischer projection formula. In Fischer projection the chiral carbon is the intersection point of the two lines crossing each other. The horizontal lines are the bonds coming forward towards the viewer (wedges) and the vertical lines are the bonds pointing downwards away from the viewer (dashed lines). Furthermore, in Fischer projection formula the carbonyl groups are placed at the top.
In Fischer projections the R and S designations are assigned to each stereocenter by applying following rules.
- 1) Draw the Fischer projection and identify the stereocenter.
- 2) Assign priorities to all four different groups using Cahn Ingold Prelog Rules.
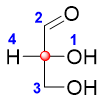
- 3) Draw a circle moving from 1 🡪 2 🡪 3.
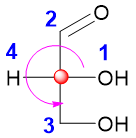
As drawn above, the lowest priority present on the horizontal axis (wedge) came before the priority 3. In such cases the designation is reversed. As the circle is making an S designation so we will reverse it and make it R.
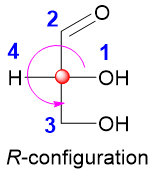
If the lowest priority is pointing backwards (present on the vertical axis, dashed line) then there is no need of reversing the designation. For example,
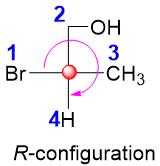
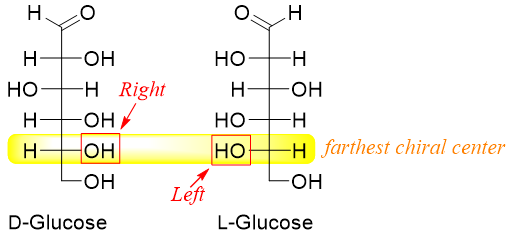
The naturally occurring carbohydrates with R-configuration are also called D-isomers while their S-configuration enantiomers are called L-isomers.
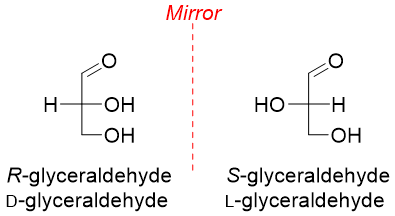
The D and L system is used to label all monosaccharides either containing single or multiple stereocenters. The D and L labels are applied to the chiral center present at the bottom of the Fischer projection, or we can say the chiral center present farthest from the carbonyl group.
In D-sugars the hydroxyl group at the farthest chiral center is present at the right hand side of the Fischer projection whereas, in L-sugars the hydroxyl group at the farthest chiral center from carbonyl group is present at the left hand side of the Fischer projection.