The Family of D-Aldoses
The Family of D-Aldoses
The family of D-aldoses consists of sugars having an aldehyde functional group at position one and a stereocenter with a hydroxyl group at the right hand side of the Fischer projection present farthest from the carbonyl group.
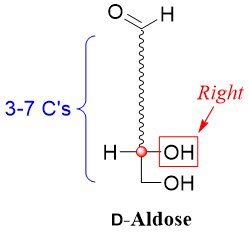
The simplest D-aldose is the D-glyceraldehyde. Using D-glyceraldehyde as a reference one can devise higher D-aldoses by placing carbon atoms bonded to hydrogen atom (-H) and hydroxyl group (-OH) between C1 and C2 of D-glyceraldehyde. For example
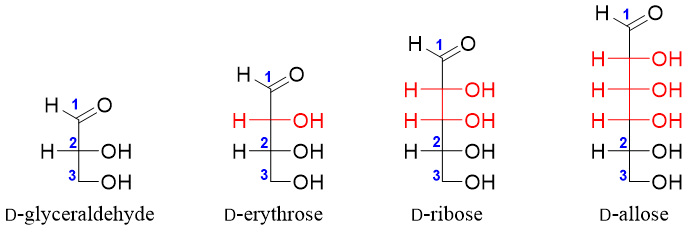
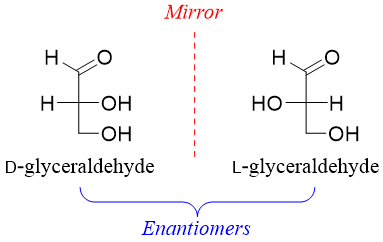
Glyceraldehyde (aldotriose) has one stereocenter thus, it has two stereoisomers (2n = 21 = 2). Both stereoisomers are enantiomers of each other.
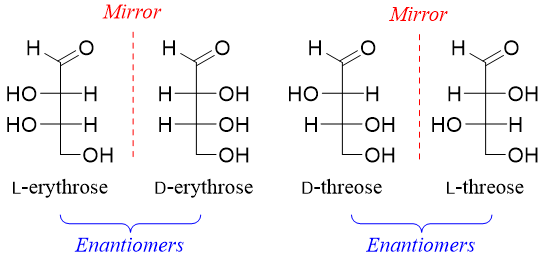
Aldotetroses have two stereocenters hence, they will have four stereoisomers (2n = 22 = 4). Two of the stereoisomers are D-erythrose and D-threose and the other two will be their enantiomers (L-erythrose and L-threose).
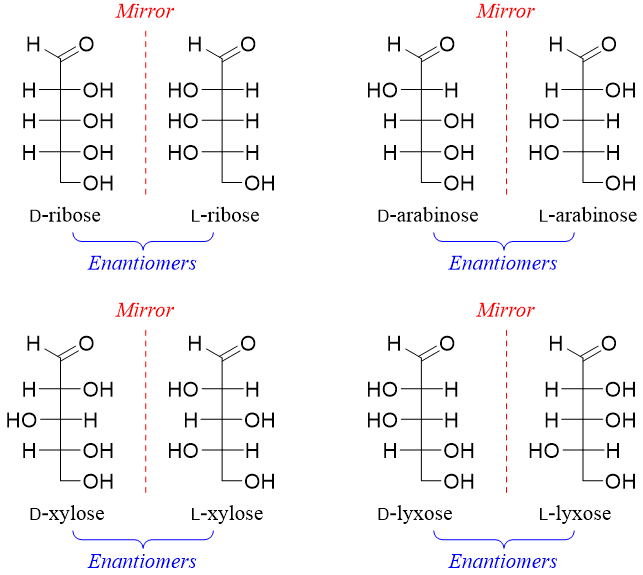
Aldopentoses have three stereocenters hence, they will have eight stereoisomers (2n = 23 = 8). Four of the stereoisomers are D-ribose, D-arabinose, D-xylose and D-lyxose and the other four will be their enantiomers (L-ribose, L-arabinose, L-xylose and L-lyxose).
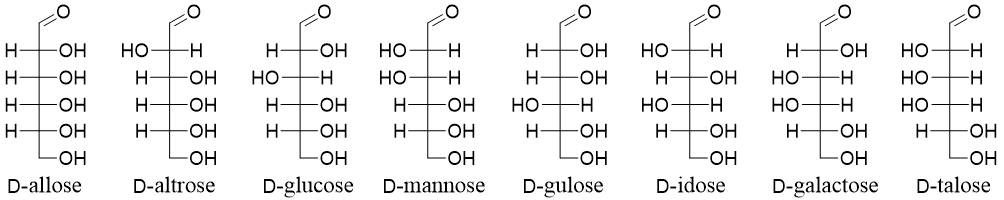
Aldohexoses have four stereocenters hence, they will have four stereoisomers (2n = 23 = 8). Eight of the stereoisomers are D-allose, D-altrose, D-glucose, D-mannose, D-gulose, D-idose, D-galactose, and D-talose and the other eight will be their enantiomers (L-allose, L-altrose, L-glucose, L-mannose, L-gulose, L-idose, L-galactose, and L-talose).
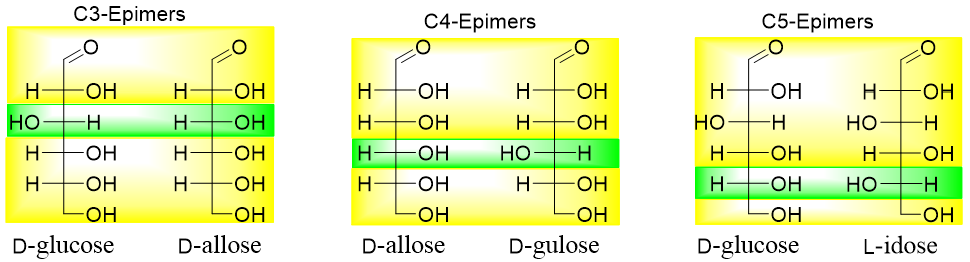
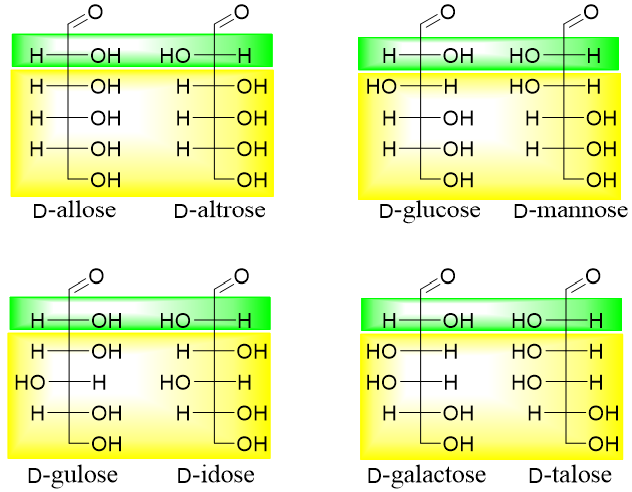
All above stereoisomers are diastereomers of each other. Those diastereomers in which the configuration around only one stereocenter is different are termed as epimers. For example, in aldohexoses following pairs are epimers of each other. Following are the C2 epimers of aldohexoses. The yellow highlighted area shows the same configurations while the green highlighted area shows different configurations.
Following are some examples of C3, C4, and C5 epimers of aldohexoses.