Cyclic forms of Monosaccharides
Cyclic forms of Monosaccharides
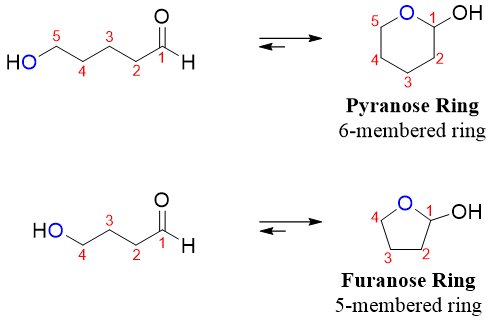
The carbonyl group and hydroxyl group of acyclic forms of monosaccharides can undergo intramolecular cyclization to form a cyclic hemiacetal containing either five or six atoms in the ring.
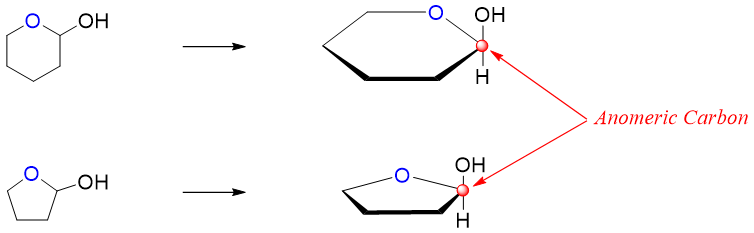
The addition of hydroxyl group to carbonyl group forms a stereocenter at the hemiacetal center. The hemiacetal carbon center is also termed as anomeric carbon.
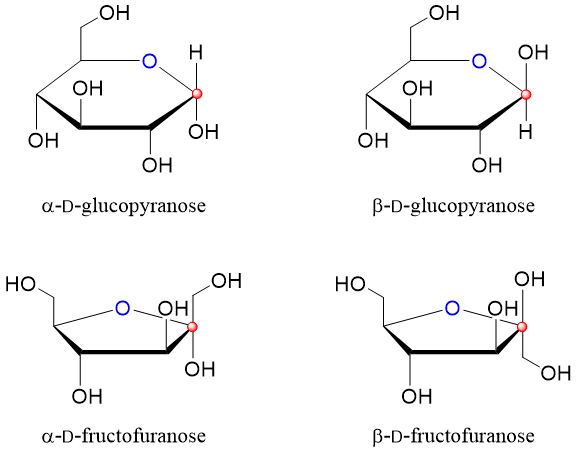
Each hemiacetal carbon gives rise to two anomers. The two anomers differ in the position of the hydroxyl group at position C1. Hence, anomers are in fact epimers of each other only differing in position of hydroxyl group at C1. Following are some examples of anomers.
Drawing cyclic hemiacetal:
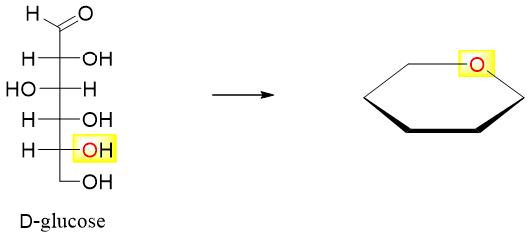
- 1) First of all, determine the stability of the ring formed by the cyclization reaction. The aldohexoses will form six membered rings while ketohexoses will form five membered rings.
- 2) In aldohexoses (D-glucose) place the oxygen atom present at the farthest stereocenter (C5) in the upper right hand corner of the six-membered ring.
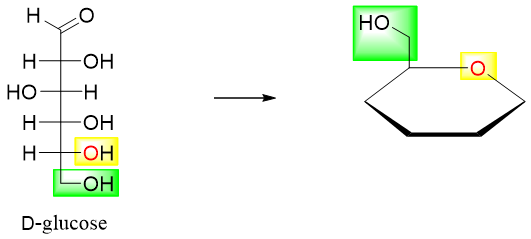
- 3) In D-glucose the -CH2-OH is drawn up whereas, in L-glucose the -CH2-OH is drawn down.
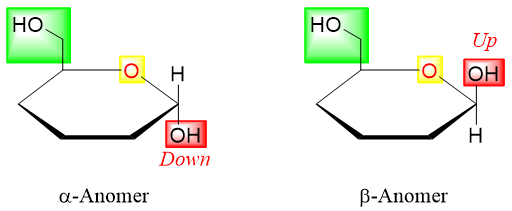
- 4) On anomeric carbon (formed from carbonyl group) if the -OH is pointing down then it is α-anomer, if it is pointing up then it is β-anomer.
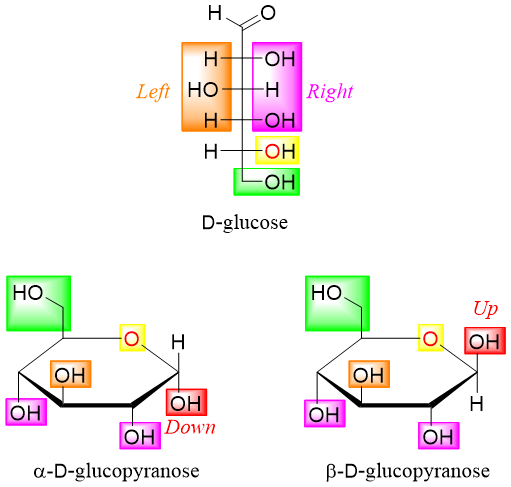
- 5) Add the remaining substituents in a clockwise The substituents present on the right hand side of the Fischer projection are drawn down on Haworth projection and substituents on the left hand side of Fischer projection are drawn up on Haworth projection.
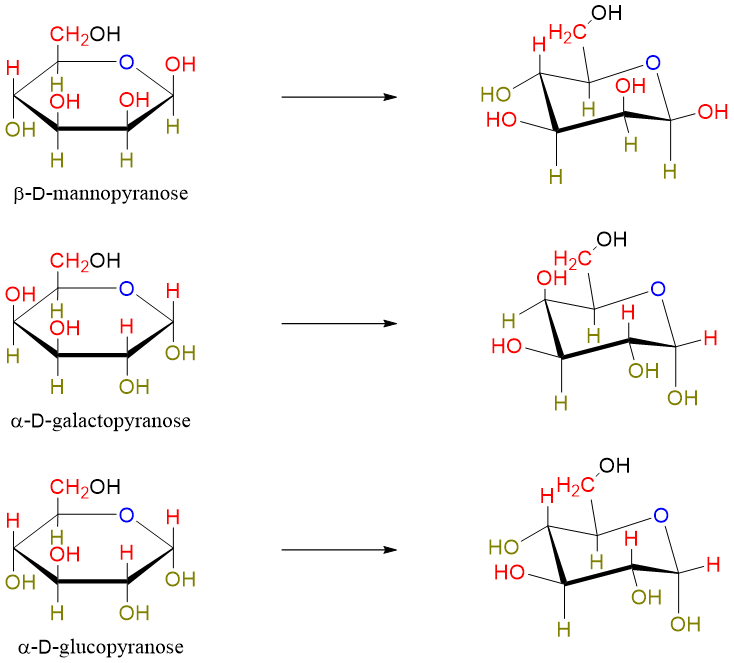
Converting Haworth projection to Chair conformation:
First draw the chair conformation and put the oxygen atom at the top right corner.

Next determine the “up” and “down” positions both on chair conformation and Haworth projection.
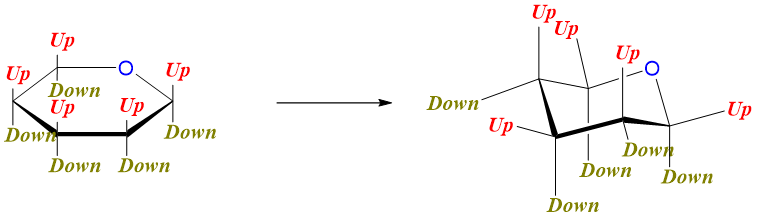
Examples: