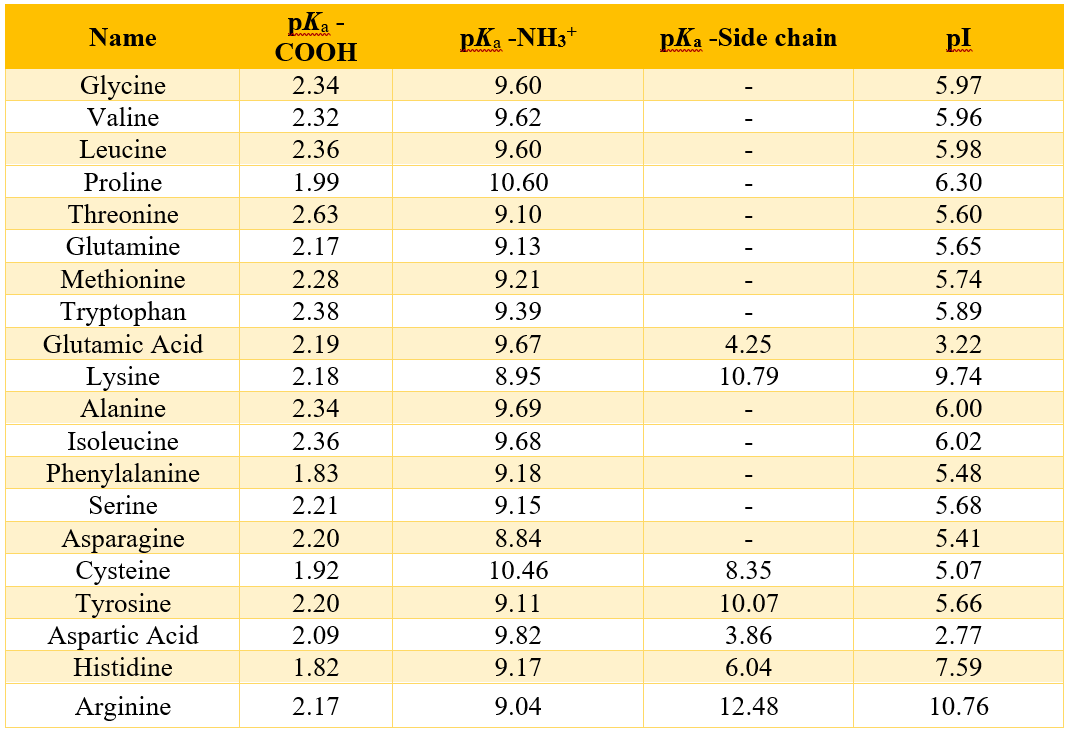
The Isoelectric Point
The Isoelectric Point
Amino acids bear different charges in solutions with different pH’s. Amino acid exists in the form of zwitterions (neutral) when it is added into neutral solutions. Whereas it gets negatively and positively charged when added into basic and acidic solutions, respectively. The pH at which the amino acid exists in the form of zwitterion is called isoelectric pH or the isoelectric point (pI).
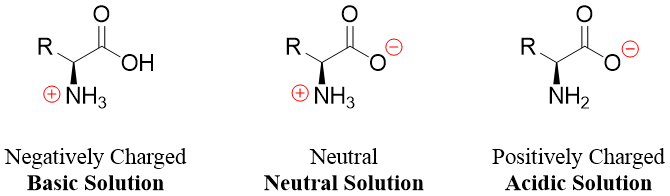
Amino acids with no ionizable side chains have the pI values equal to the average of both pKa values of the -COOH group and the -NH3+ group. For example, the valine has a pI value of 5.97 which is the average of pKa 2.32 and 9.62.
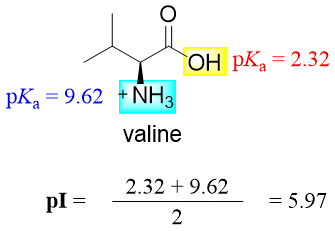
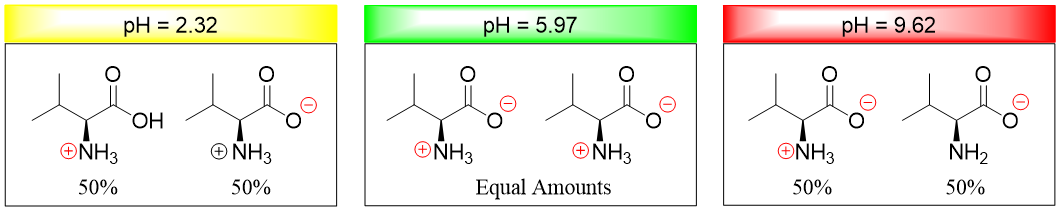
The pI is taken as the average of two pKa values because at this point the number of positively charged groups equals the number of negatively charged groups. This can be further elaborated as when the pH of the solution is 2.32 half of the molecules of valine are neutral (contain -COO- and -NH3+ groups) and half of the molecules are positively charged (contain -COOH and -NH3+ groups). When the pH is increased the number of -COO- groups increases hence more neutral valine molecules are formed. Whereas when the pH of the solution is 9.62 half of the molecules of valine are neutral (contain -COO- and -NH3+ groups) and half of the molecules are negatively charged (contain -COO- and -NH2 groups). When the pH is decreased the number of -NH3+ groups increase hence more neutral molecules are formed. At the pI stage the number of molecules containing -NH3+ groups become equal to the number of molecules containing -COO- groups.
The pI of amino acids containing ionizable side chains is calculated by taking the average of pKa values of similar ionizing groups. For example, the pI value of aspartic acid is 2.975 and pI value of arginine is 10.76.
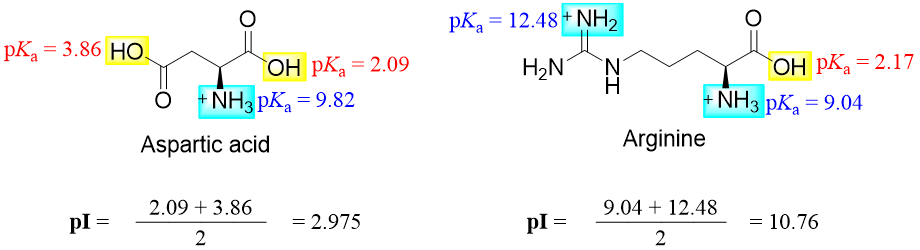
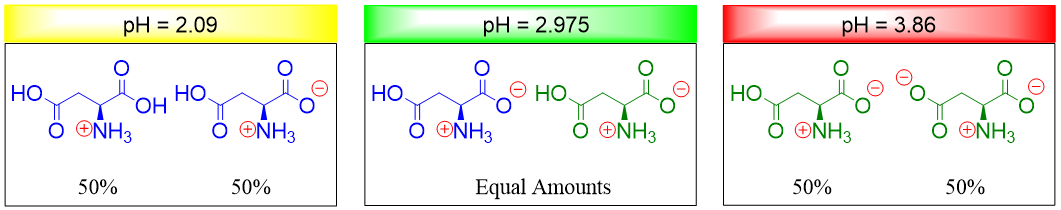
At pH 2.09 half of the aspartic acid molecules are neutral and half of the molecules are positively charged. Hence, when pH is increased the number of neutral molecules increases. At pH 3.86 half of the aspartic acid molecules are neutral and half of the molecules are negatively charged. Hence, when pH is decreased the number of neutral molecules increases. Thus, at pH 2.975 (isoelectric point) the number of positively charged groups equals the number of negatively charged groups.
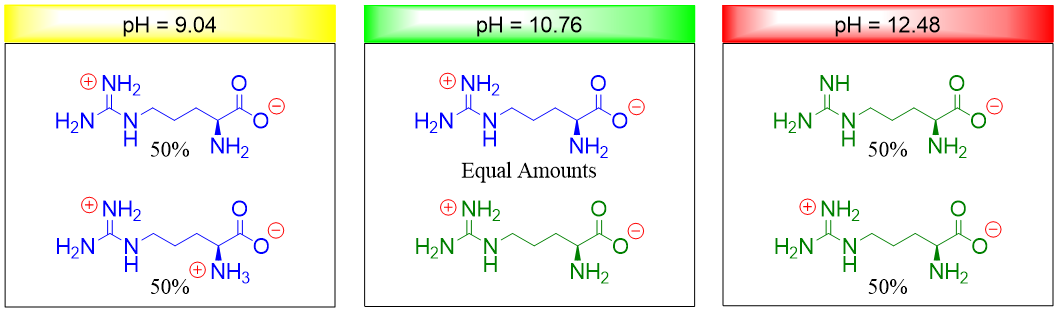
Similarly, at pH 9.04 half of the arginine molecules are neutral and half of the molecules are positively charged. Hence, when pH is increased the number of neutral molecules increases. At pH 12.48 half of the arginine molecules are neutral and half of the molecules are negatively charged. Hence, when pH is decreased the number of neutral molecules increases. Thus, at pH 10.76 (isoelectric point) the number of positively charged groups equals the number of negatively charged groups.
Those amino acids which contain acidic side chains have isoelectric points at lower pH values because these acidic side chains provide extra negative charges. Therefore, the neutral forms of these amino acids exist under more acidic conditions. On the other hand, amino acids which contain basic side chains have isoelectric points at higher pH values because these basic side chains provide extra positive charges. Therefore, the neutral forms of these amino acids exist under more basic conditions. Following table shows the pI values of all standard amino acids.