Synthesis of Amino Acids
Synthesis of Amino acids
Amino acids are abundantly found in nature. Although they can be synthesized in laboratories by using different methods. Some of these methods are described below.
Hell–Volhard–Zelinskii Reaction followed by reaction with Ammonia:
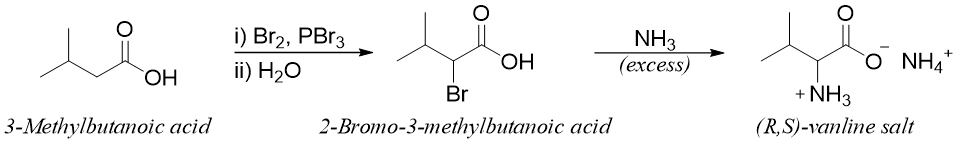
This is the oldest method for the synthesis of amino acids. In this reaction the carboxylic acid is treated with Br2 and PBr3 to form α-bromo acid. The α-bromo acid on reaction with ammonia undergoes SN2 reaction and forms α-amino acid.
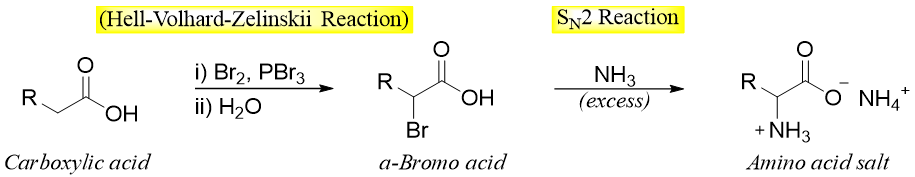
In this reaction the ammonia is used in excess because the presence of a carboxylic group reduces the nucleophilicity of ammonia by converting it into ammonium ion. Following reactions show the synthesis of valine by using this method.
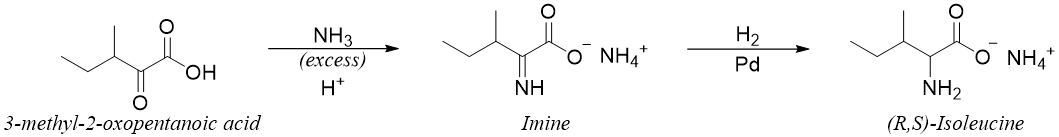
Reductive Amination of α-ketoacids:
When α-ketoacid is reacted with ammonia an imine is formed. The imine is further reduced to amine using reducing agents which do not reduce the carboxylic group like H2 gas in the presence of palladium catalyst. This reaction is a one pot reaction and does not require the isolation of the imine intermediate.
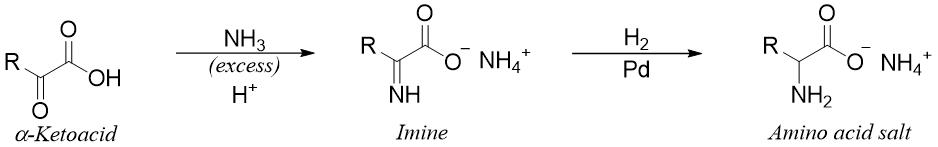
Following reaction shows the synthesis of isoleucine.
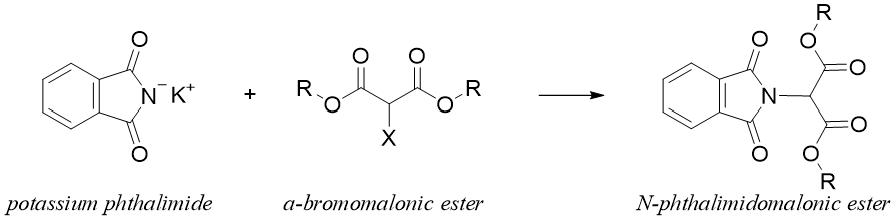
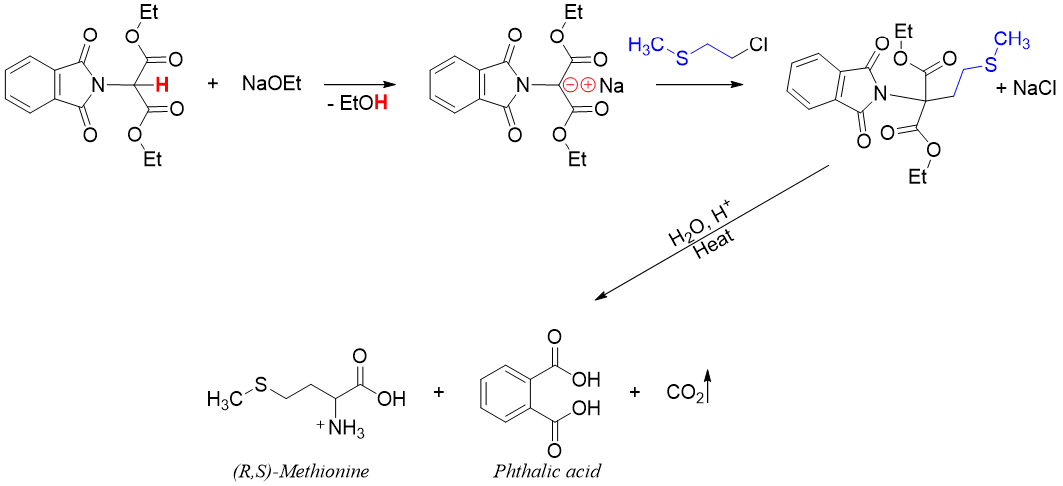
Gabriel-Malonic Ester Synthesis:
Amino acids can be synthesized in good yields via the N-phthalimido malonic ester synthesis method. This method includes both Gabriel synthesis and malonic ester synthesis. In this reaction N-phthalimido malonic ester is formed by reacting potassium phthalimide with α-Bromo malonic ester via SN2 reaction.
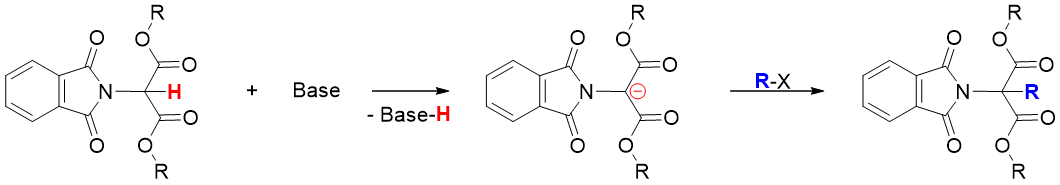
Next, N-phthalimido malonic ester is treated with a base which abstracts the acidic proton present between two carbonyl groups. The resulting carbanion is reacted with alkyl halide via SN2 alkylation reaction.
Finally, the alkylated N-phthalimido malonic ester is heated and hydrolyzed to form amino acid, phthalic acid and CO2 gas.
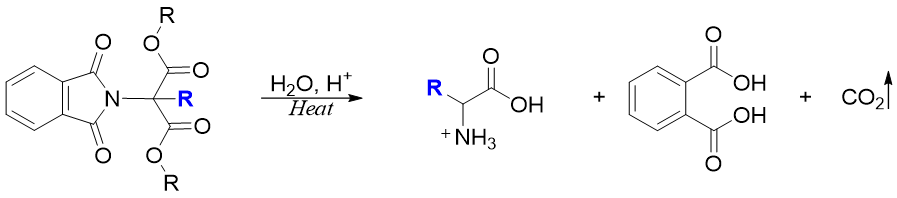
This method is used to synthesize those amino acids which are formed in poor yields by direct amination of α-halo acids. For example, methionine is synthesized through this method.
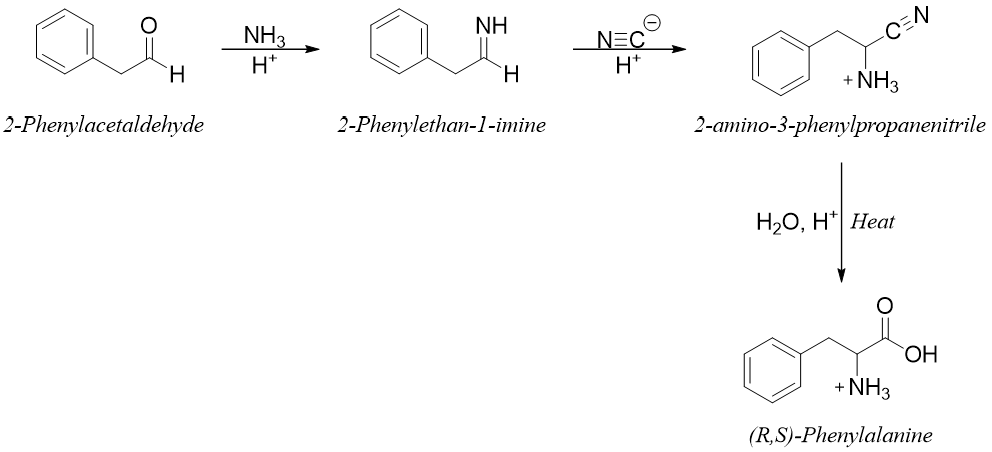
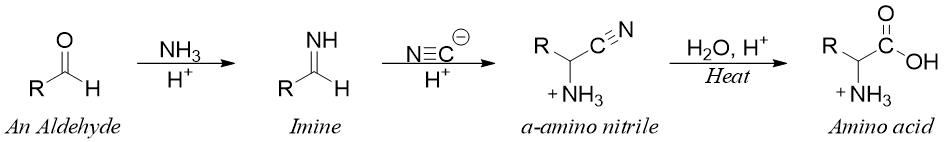
The Strecker Synthesis:
This is the first known method used for the synthesis of amino acids. This method can be used to synthesize a large number of amino acids. In this reaction an imine is formed by the reaction of aldehyde and ammonia followed by addition of cyanide ion to form an α-amino nitrile intermediate. The α-amino nitrile upon hydrolysis gives corresponding amino acid.
The Strecker synthesis of phenylalanine is shown below.