Levels of Protein Structures
Levels of Protein Structures
Proteins are classified into four levels of structures. i) Primary structure simply explains the sequence of amino acids along with any disulfide linkages present in protein. ii) Secondary structure explains how segments of the protein modify into regular patterns. iii) Tertiary structure explains how the entire protein molecule arranges into a three-dimensional structure. iv) Quaternary structure explains how two or more proteins interact to form an aggregate structure.
Primary Structure:
Primary structures show the sequence of amino acids bonded to each other through peptide bonds. Hence, peptide bond is the principal element of primary structures. Primary structures also explain any disulfide linkages present in a protein. In primary structures the rotation about the sigma bonds is allowed hence the entire peptide chain can twist and attain different arrangements. Whereas the rotation about amide bonds is restricted. The twisting of the peptide chain results in the formation of secondary structures.
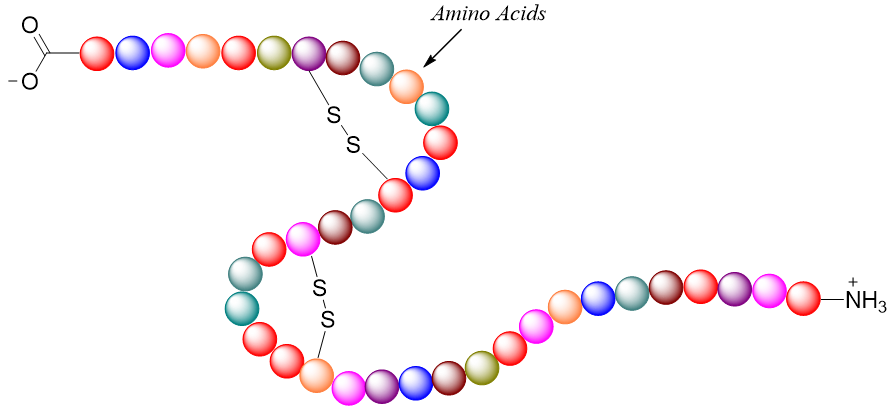
Secondary Structure:
The secondary structure explains the repetitive conformations attained by the segments of the protein chain. The factors responsible for the secondary structures of a protein segment are the regional planarity of the peptide bond which stops the amide bond rotation, formation of hydrogen bondings between the amino acids to attain stability, and separation between the side chains of amino acids to reduce repulsion between similar charges. Two stable arrangements formed in secondary structures are α-helix and β-pleated sheets.
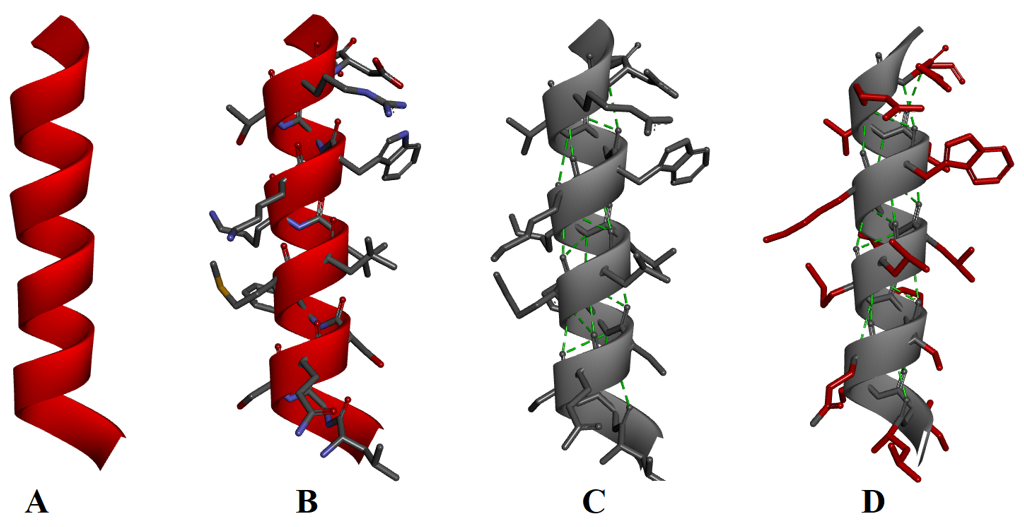
α-Helix:
An α-helix is formed when the backbone of the peptide chain twists into a right hand coil. In α-helix each turn of the coil constitutes about 3.6 amino acid residues. All the C=O and N-H bonds are present along the axis of the α-helix i.e., N-H and C=O bonds point in opposite directions to each other. Hydrogen bonds are formed between the C=O and N-H groups present four amino acids away. All hydrogen bonds formed are along the axis of the α-helix. The side chains of the amino acid residues extend outward from the core of the α-helix.
β-Pleated Sheets:
The β-pleated sheets are formed by the two or more peptide strands lined up side-by-side. In β-pleated sheets the N-H and C=O groups lie in the plane of the sheet and the hydrogen bonds are formed between them. The side chains of the amino acids are oriented below and above the plane of the sheets in an alternate fashion. β-pleated sheets are commonly formed by amino acids containing small alkyl side chains like alanine and glycine as the large side chains can cause steric hindrance preventing the chains to come close to each other. The peptide chains in β-pleated sheets can be oriented either in the same direction (parallel β-pleated sheets) or in opposite directions (antiparallel β-pleated sheets). β-pleated sheets are represented by a flat helical ribbon.
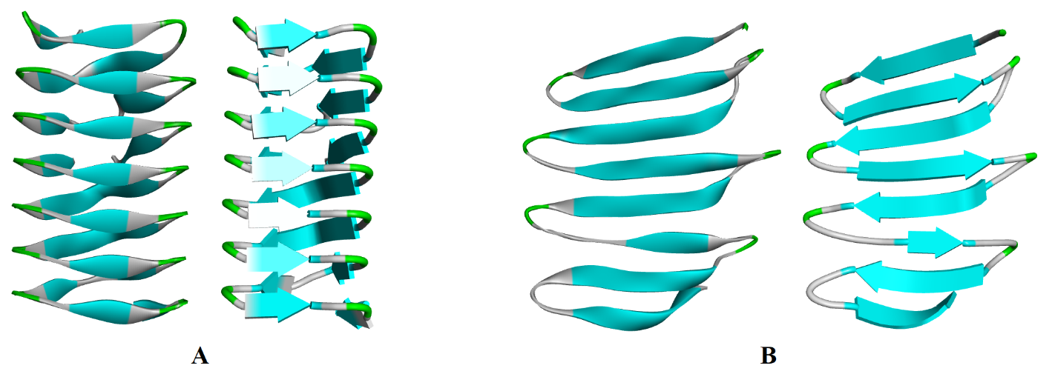
(A) Parallel β-pleated sheets and (B) Antiparallel β-pleated sheets
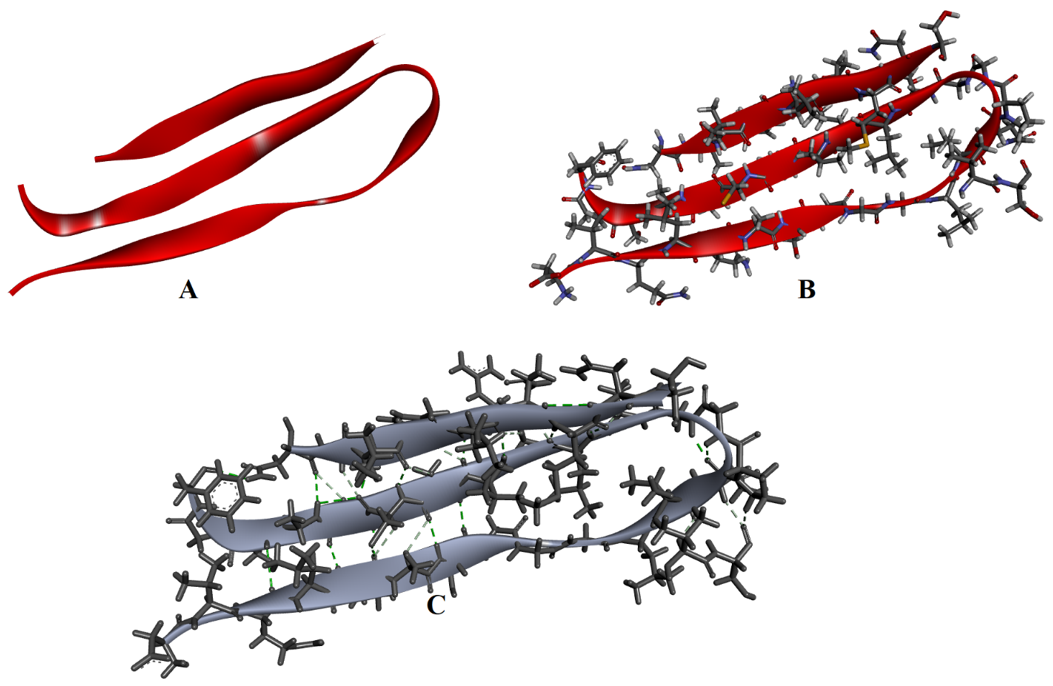
(A) β-pleated sheets, (B) Amino acids shown in β-pleated sheets, and (C) Hydrogen bonding interactions (green) between the strands.
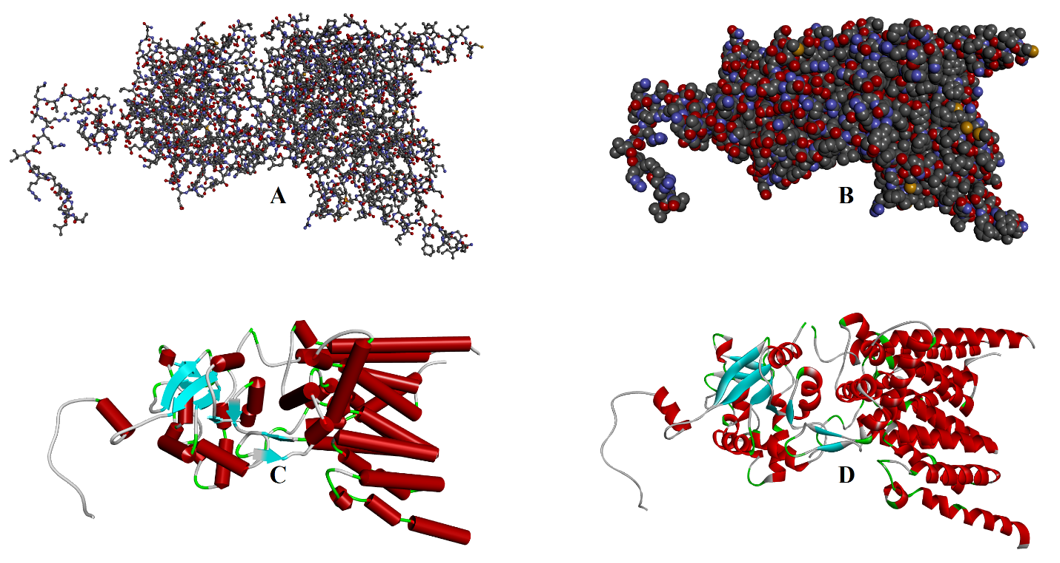
Tertiary Structure:
At the tertiary structure level the peptide chain adopts a three-dimensional shape. In this structure the peptide chain folds and generates different intramolecular interactions which minimizes the overall energy of the protein. In the folding process, the highly charged and polar side chains of amino acids are placed at the outer surface of the protein. These polar side chains interact with polar solvents like water and make protein soluble in it. Whereas the non-polar side chains of amino acid residues are placed in the interior part of the protein. The hydrophobic interactions between these non-polar side chains stabilizes the protein. In addition, electrostatic interactions between the charged groups like -COO- and -NH4+ also stabilizes the protein three-dimensional structure. Furthermore, disulfide linkages are the only covalent bonds which are formed between the cysteine residues and are responsible for stabilizing the protein structure.
Quaternary Structures:
In quaternary structure two or more proteins aggregate together to form a complex three-dimensional structure. Each protein is called a subunit of the overall complex protein. The protein containing a single subunit is called monomer, having two subunits is dimer, having three subunits is trimer, and having four subunits is called tetramer. The subunits contained by a protein can be the same or different.
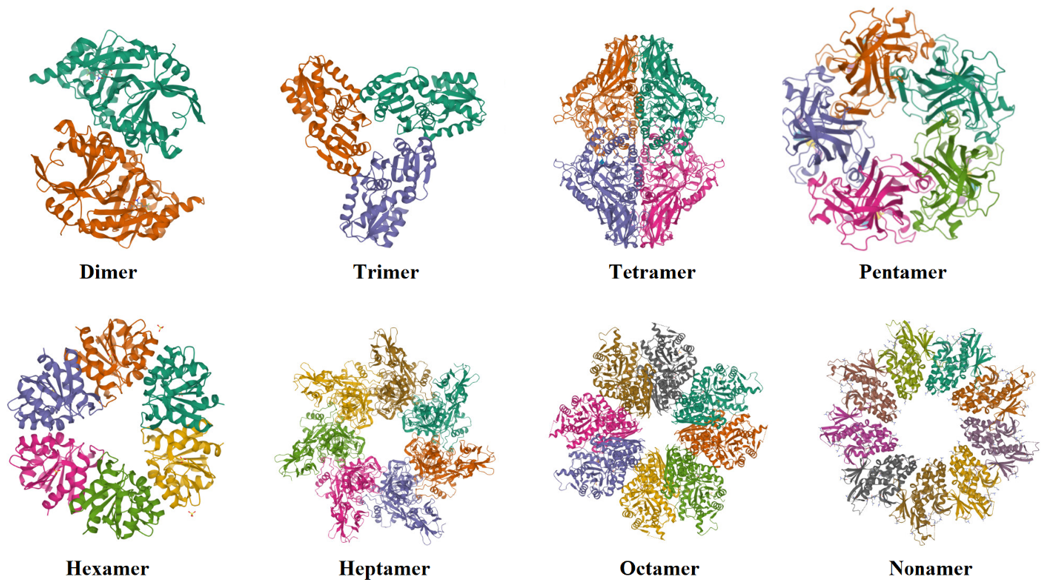
Quaternary structures of proteins containing different numbers of subunits.
