Protein Denaturation
Protein Denaturation
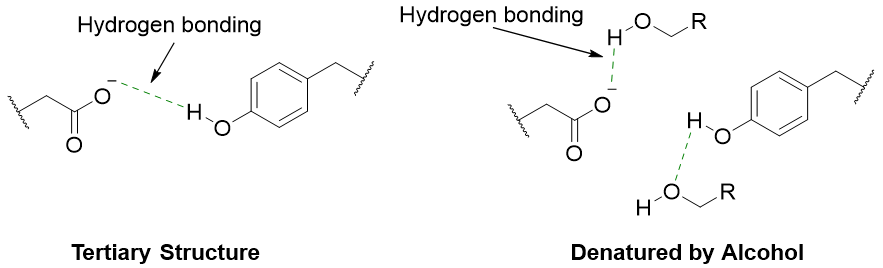
The process of destroying the secondary, tertiary, and quaternary structures of proteins is called protein denaturation. A protein in its natural state has a highly organized three-dimensional structure. This structure is attained by different interactions between the amino acid residues including disulfide linkages, hydrogen bondings, electrostatic force of attraction, and other Van Der Waals forces. Natural proteins with these interactions have a specific conformation and are responsible for many functions. If these interactions are removed / broken down, then the protein will unfold and lose its three-dimensional shape and is termed as denatured. Following figure shows the denaturation of protein.
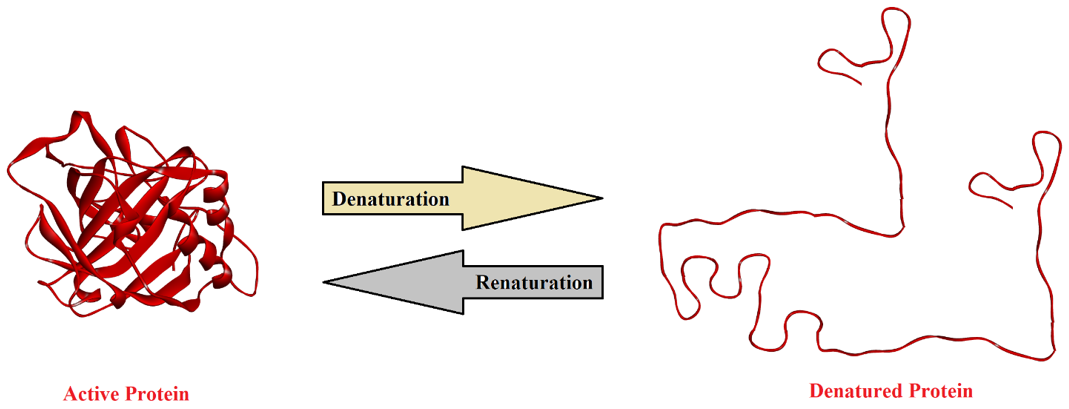
The denaturation of different proteins results in following changes.
Loss in biological activity:
Various proteins like enzymes have some specific conformation which makes the protein active in catalyzing specific reactions. When these enzymes are denatured, their structures are destroyed hence, they lose their functions. Some viral enzymes when treated with alkali or subjected to heat become inactive and they no longer cause a disease.
Susceptible to enzymatic hydrolysis
Proteins in their denatured state are more exposed and can easily be hydrolyzed by enzymes at their peptide bonds. Hence the proteins in its unfolded states are more susceptible to digesting enzymes.
Changes in solubility:
When neutral salts are added to protein solution it results in denaturation of the protein. This process is accompanied by the precipitation of the proteins. This shows that the denatured proteins are insoluble in water.
Crystal property:
Many proteins like globular proteins form crystal structures. Once denatured these proteins lose their ability of crystal formation.
There are many factors which are responsible for the denaturation of proteins. These factors can be physical; temperature, pressure, irradiation, sound waves, and surface forces and chemical ; pH, solvents, and organic solutes. Few of them are discussed below.
Urea:
Urea is mostly used for the denaturation of proteins. Urea denatures protein either by directly interacting with the protein and making it soluble in water or indirectly by changing the behavior of water which reduces the hydrophobic interactions between the residues of protein. In direct interaction the urea binds to the protein and stabilizes the denatured state of the protein. Hence, urea can denature the protein by solvating the protein's backbone through hydrogen bond interactions, forming electrostatic interactions with the hydrophilic residues of protein, and forming hydrophobic interactions with hydrophobic residues of the protein.
Heat:
Heat is one of the most familiar denaturing factors for proteins. When the solution of protein is subjected to heat the proteins will coagulate. Some proteins when cooled regain their original state while some are irreversible like egg white. At high temperatures, the kinetic energy of the protein increases and it vibrates violently. These vibrations result in the breakdown of hydrogen bond interactions and other hydrophobic interactions resulting in the unfolding of the protein.
pH:
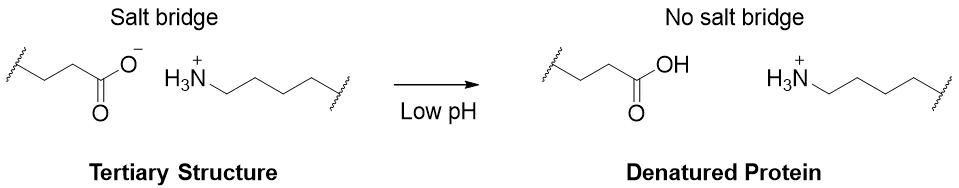
When a protein is subjected to low pH the carboxylic group of the side chain gets protonated and the ionic charge ability is lost. This results in conformational changes leading to denaturation.
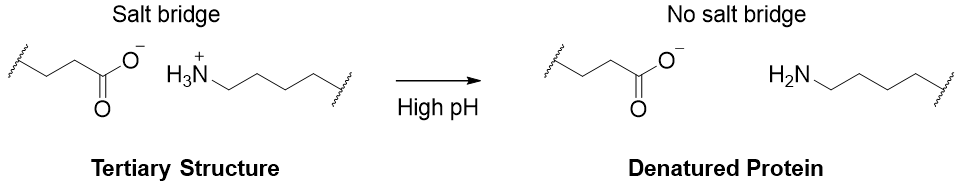
Whereas, at high pH the amino groups are deprotonated hence, losing its ionic character, and leading to denaturation.
Different proteins behave differently at different pH levels. Some proteins at low pH retain their natural conformation while some get denatured and vice versa. This shows that change in pH can cause changes in the conformation of proteins and denature them. Following are some examples of proteins and their behavior at different pH levels.
Lysozyme, β-lactoglobulin, and ribonuclease remain unaffected at low pH.
Yeast glyceraldehyde-3-phosphate dehydrogenase changes its activity at low pH.
Hemoglobin and Myoglobin are stable at high pH.
Organic solvents:
The denaturation of proteins can take place when organic solvents like alcohols and acetone are added to its aqueous solution. These organic solvents disrupt the hydrophilic and hydrophobic interactions between the polar and non-polar side chains of residues leading to denaturation.