Resolution of Amino Acids
Resolution of Amino acids
Enantiomers cannot be separated by using ordinary methods like filtration, distillation, crystallization, normal chromatography etc. as enantiomers have the same physical properties. On the other hand, diastereomers have different physical properties therefore they can be separated by general separating methods. Naturally, amino acids exist in S-configuration. Whereas the synthesis of amino acids results in the formation of both enantiomers. The separation of these two enantiomers is termed as resolution of racemic mixture. The oldest and still applied method for resolving a racemic mixture is to convert the enantiomers into its corresponding diastereomers. Diastereomers can be separated using ordinary separating methods. This method can be performed in three steps.
- a) Formation of Diastereomers:
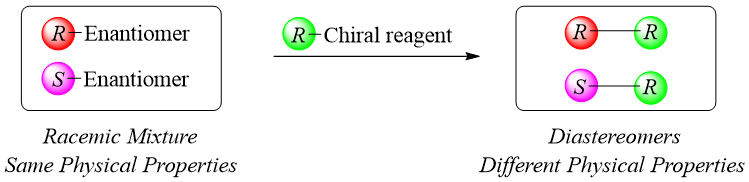
- b) Separation of Diastereomers:
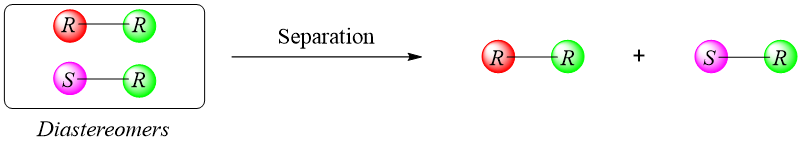
- c) Separation of pure Enantiomer:
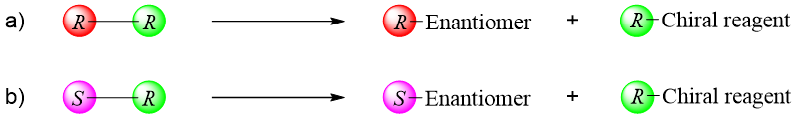
Following scheme shows the separation of a racemic mixture of valine. First the racemic mixture of valine is reacted with acetic anhydride to form a racemic mixture of N-acetyl amino acids. This step is necessary to protect the -NH2 group of amino acids.
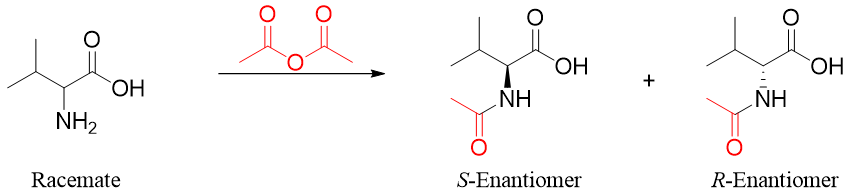
The enantiomers are still not separable therefore they are further treated with enantiopure chiral reagents like (R)-α-methylbenzylamine. This will result in the formation of diastereomeric salts.
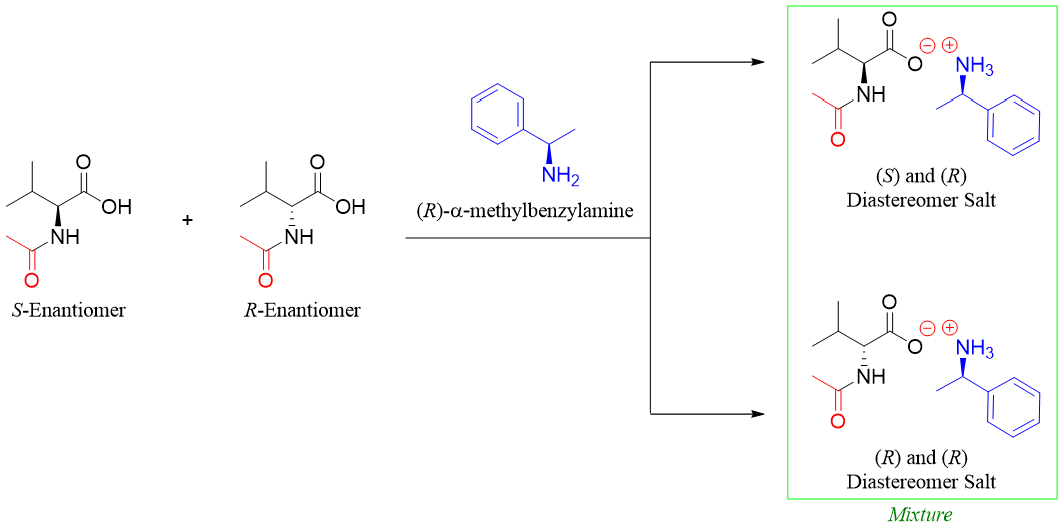
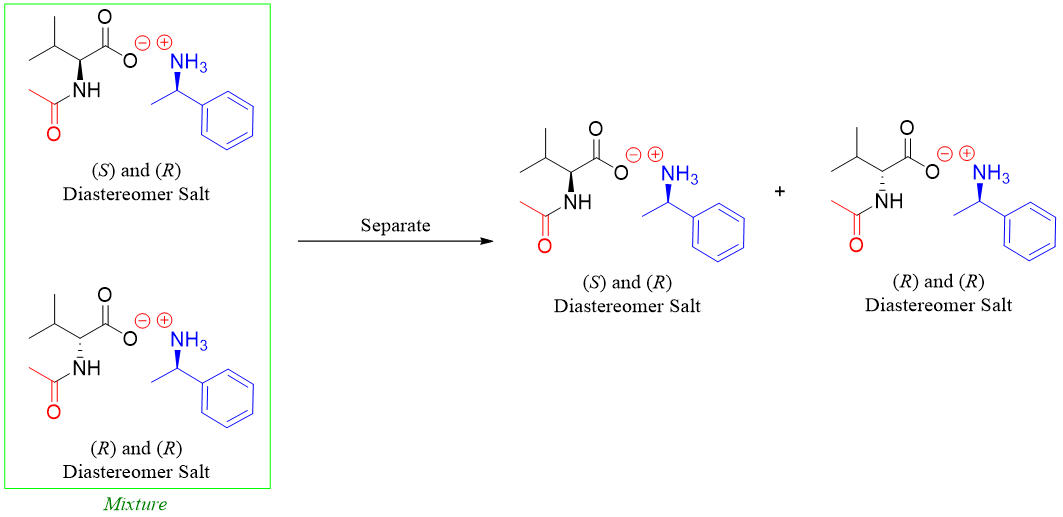
Next the diastereomers formed are separated from each other.
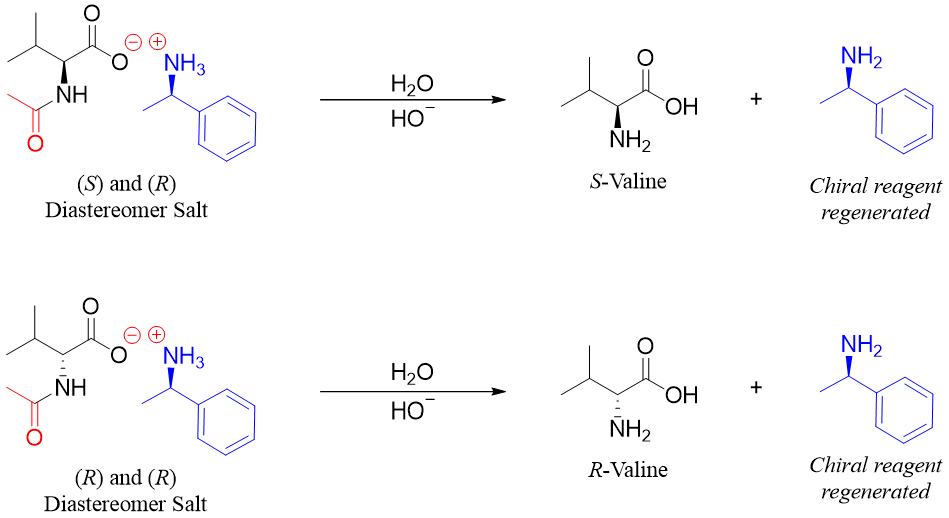
In the last step the enantiomers of valine are regenerated by hydrolysis reactions.
Another strategy employed for the separation of amino acids is to react the racemic mixture of amino acids with enzymes. Enzymes function as chiral reagents. For example, when the racemic mixture of N-acetyl alanine is treated with acylase enzyme the amide bonds are hydrolyzed. The acylase will only hydrolyze the amide bond of S-enantiomer of alanine.
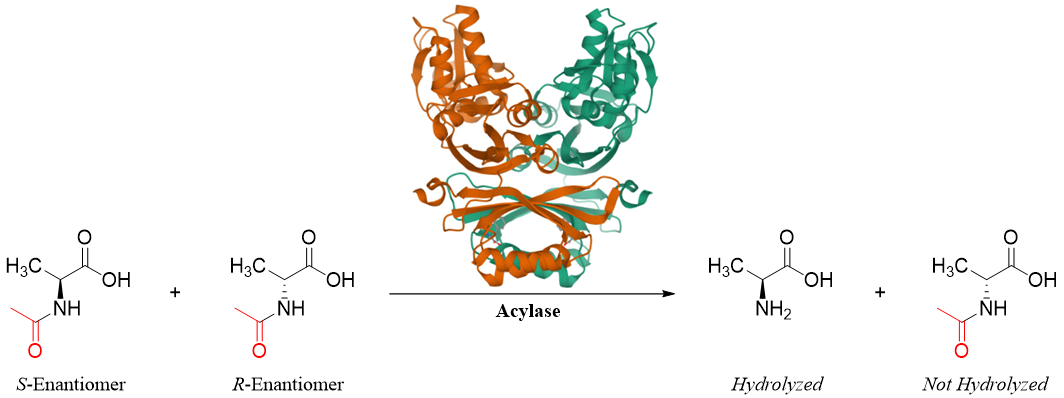
The two products formed can be separated easily. Since the enzyme is selectively reacting with a single enantiomer therefore this type of separation technique is termed as kinetic resolution.
