Peptide Sequencing
Peptide Sequencing
The structure of peptide and protein can be determined by finding the types of amino acids present in it, the number of each amino acid present in it, and the sequence of amino acids in the peptide chain. The types and number of amino acids can be determined by amino acid analyzer.
Amino Acid Analyzer:
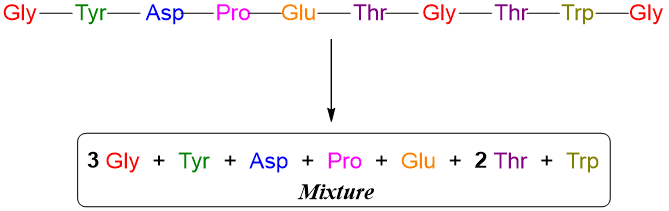
In this technique the types and number of amino acids are determined. This technique starts with treating the peptide chain (or protein) with peroxyformic acid. This results in the breakdown of any disulfide linkages present in the peptide chain(s). Next it is treated with hydrochloric acid (HCl) for 24 hours. This acidic treatment will hydrolyze the peptide bonds and form individual amino acids. Next the mixture of amino acids is separated by performing high performance liquid chromatography (HPLC). HPLC is a special type of column chromatography, and it separates the amino acids from each other on the basis of the polarity differences. This process allows us to identify each amino acid and determines the amount of each amino acid. For example, the hydrolysis of the following peptide chain will form a mixture of following amino acids.
The mixture formed is separated from each other using HPLC. Following figure depicts the spectrum formed by the amino acid residues. This spectrum is compared with the standard spectrum of amino acids. The intensity of absorption peaks shows the concentration of each amino acid whereas the retention time shows the type of amino acid.
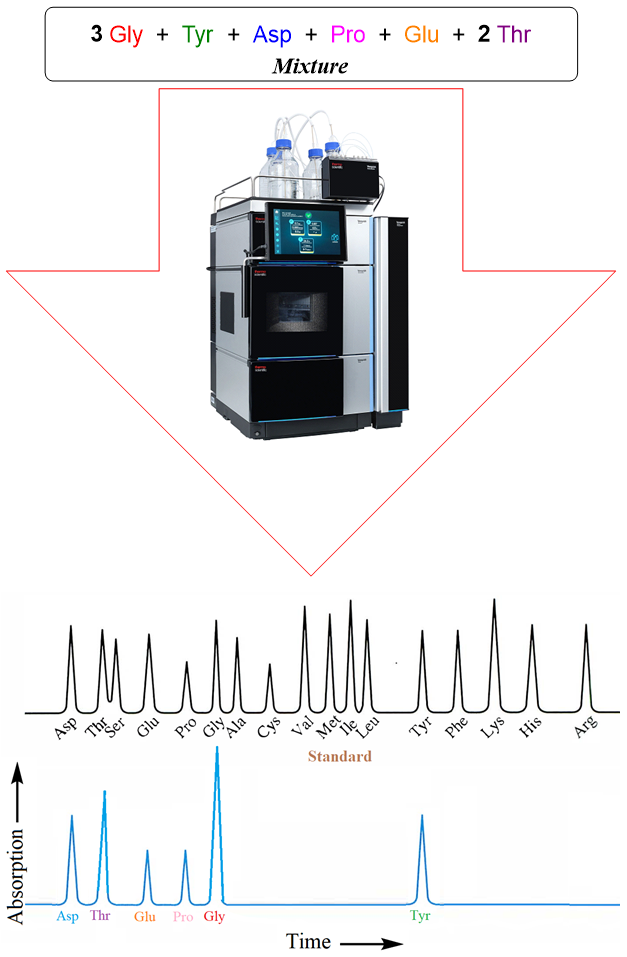
This technique only enables us to determine the type and amounts of each amino acid present in the peptide chain. Whereas the sequence of the amino acids cannot be determined by this process.
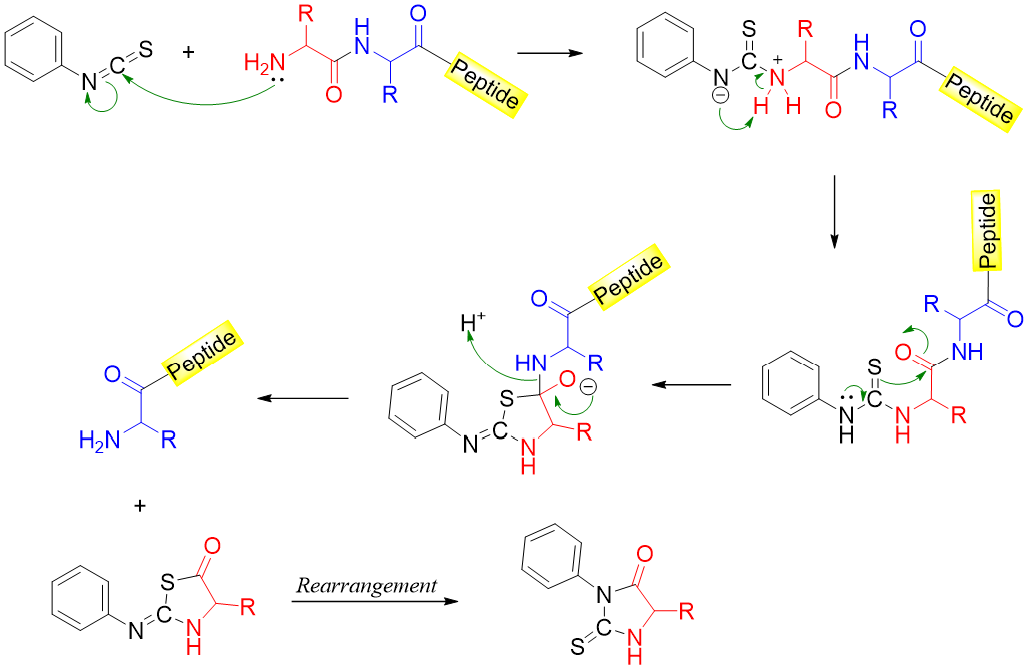
The Edman Degradation:
The Edman degradation process is used to determine the N-terminal amino acids of a peptide chain. In this process the amino acids present at the N-terminus are cleaved and identified one by one. The process is repeated until the entire amino acid sequence is measured. In this process the -NH2 group of the amino acid present at N-terminus is reacted with phenyl isothiocyanate. This results in the formation of N-phenylthiohydantoin (PTH) and a peptide chain with one less amino acid.
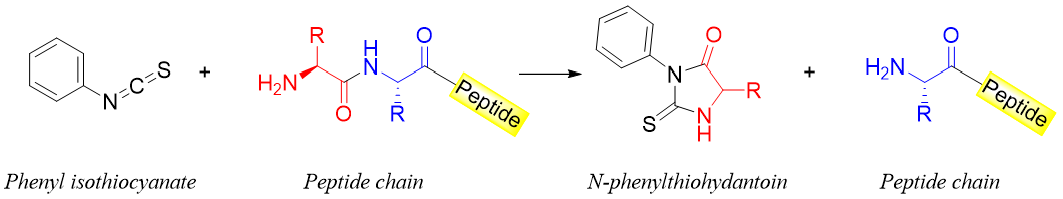
Each N-phenylthiohydantoin derivative obtained is characterized and identified. The new peptide formed after Edman degradation has a new amino acid at the N-terminus hence, the process can be repeated. The mechanism of the Edman degradation reaction is shown below.
Partial Hydrolysis of Peptides:
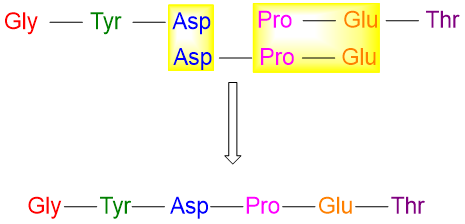
Another method for determining the sequence of a peptide chain is by doing partial hydrolysis of it. In this method some of the peptide bonds are broken in the peptide chain. This forms small fragments of peptide chains in random fashion. These small fragments are then compared and sequenced to measure the sequence of complete peptide chains. For example, when the peptide chain with following amino acids is partially hydrolyzed it forms the following three fragments.

Next all three fragments are compared, and the sites of overlap are determined.
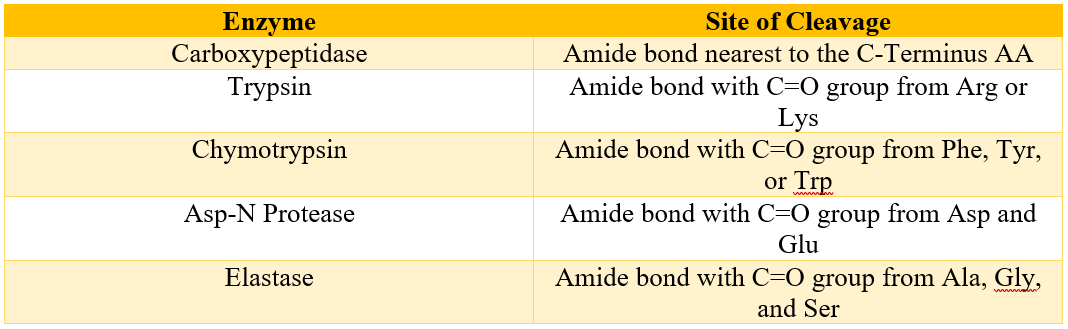
Peptide chains can also be selectively hydrolyzed at specific points using different enzymes. Following table shows some examples of enzymes and their sites of cleavages.