Peptide Synthesis
Peptide Synthesis
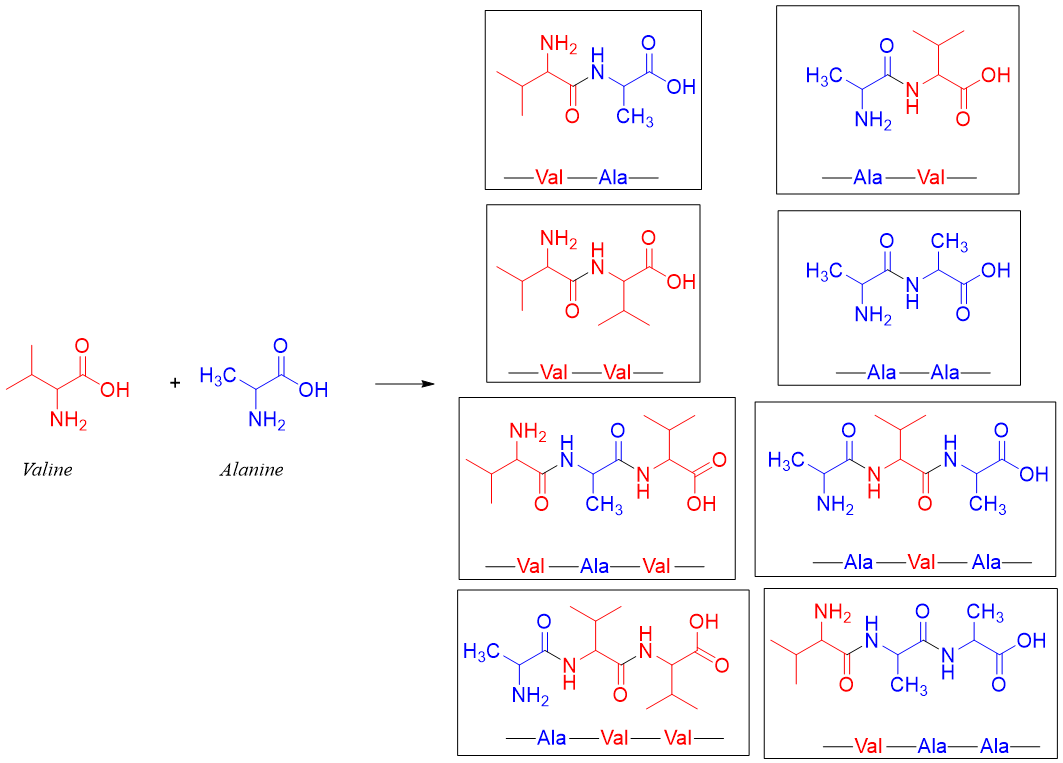
As discussed in the introduction of peptides the synthesis of specific peptide chains is a complicated process. For example, if the desired peptide is Val-Ala then it can be synthesized from valine and alanine. But the reaction of these two amino acids can result in many different peptides. Some of these peptides are shown below.
To overcome this problem the -COOH group of valine can selectively be joined to the -NH2 group of alanine to form dipeptide, Val-Ala. For this the -NH2 group of valine is first protected by reacting it with di-tert-butyl dicarbonate or 9-fluorenylmethyl chloroformate. This will convert the -NH2 group of valine to an amide bond.
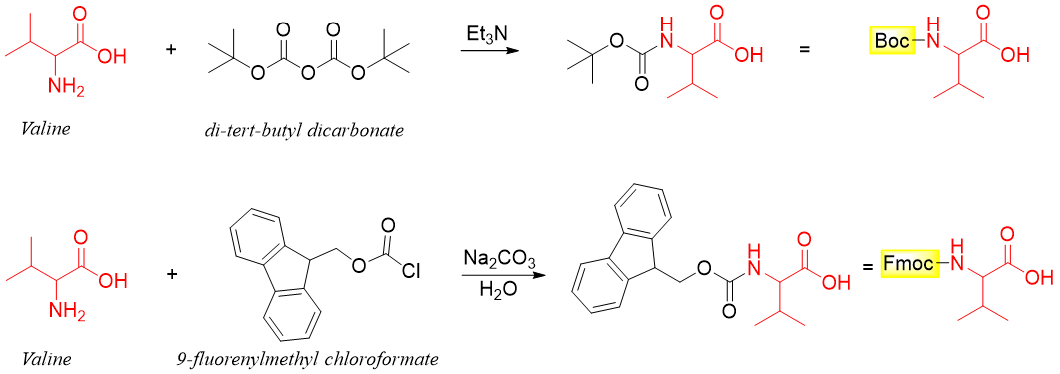
In the above scheme two protecting groups are shown. The Boc- protecting group can be removed by reacting it with an acid like trifluoroacetic acid (CF3CO2H), HBr or HCl while, the Fmoc- protecting group can be removed by treating it with base like amines or ammonia. The selection of these two groups depends upon the sensitivity of protected groups towards acidic and basic mediums.
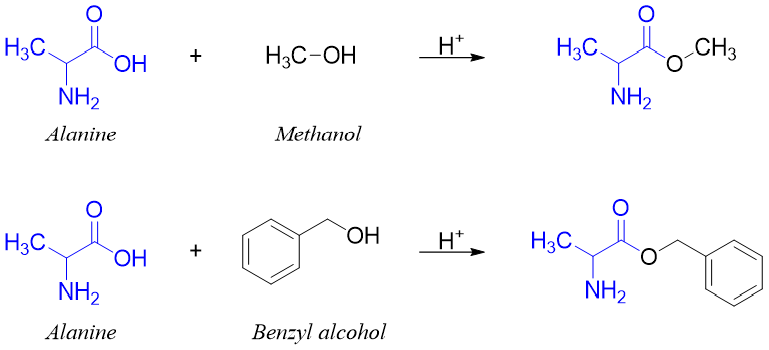
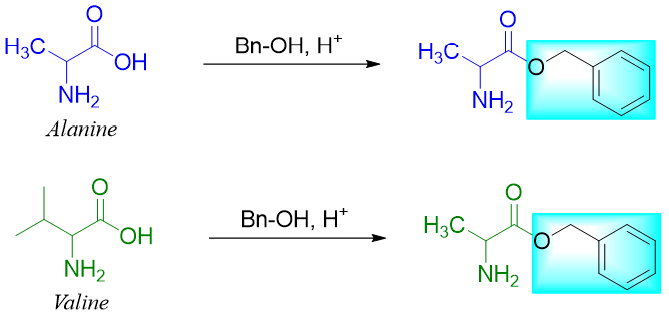
Next, the carboxylic group of alanine is protected by reacting it with alcohol in acidic medium and converting it to corresponding ester.
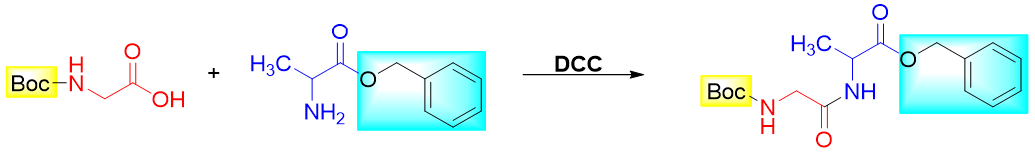
Next, the two protected amino acids are reacted with each other to form the amide bond. This reaction is assisted by using Dicyclohexylcarbodiimide (DCC). DCC is a coupling reagent and is commonly used to form an amide bond.
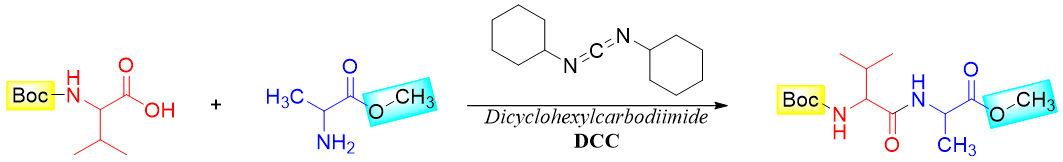
The DCC makes the hydroxyl group of -COOH a good leaving group, hence, activates the carbonyl group towards nucleophilic attack. Following is the mechanism of amide formation in the presence of DCC.
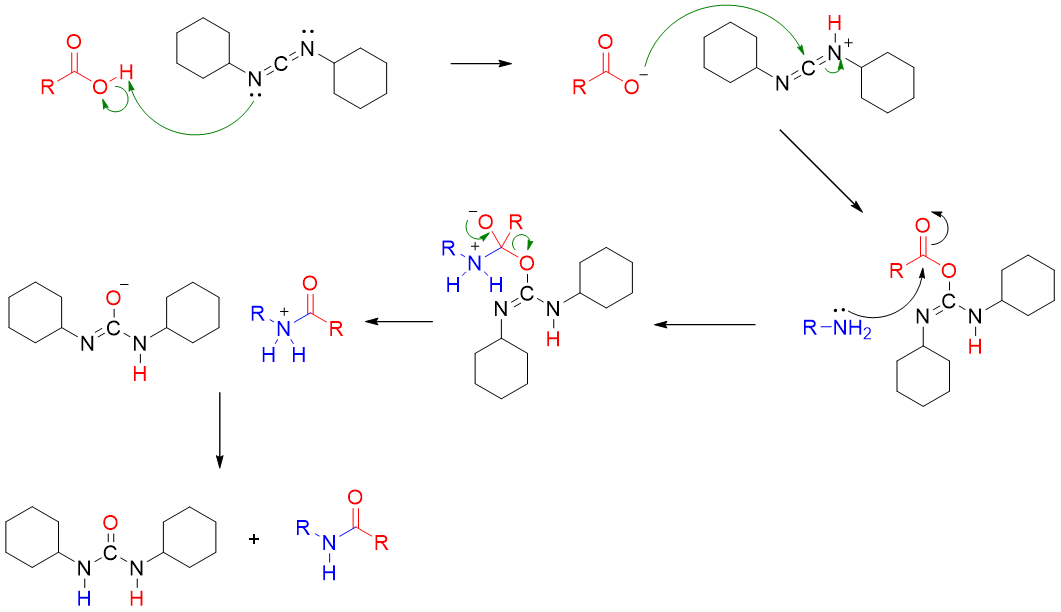
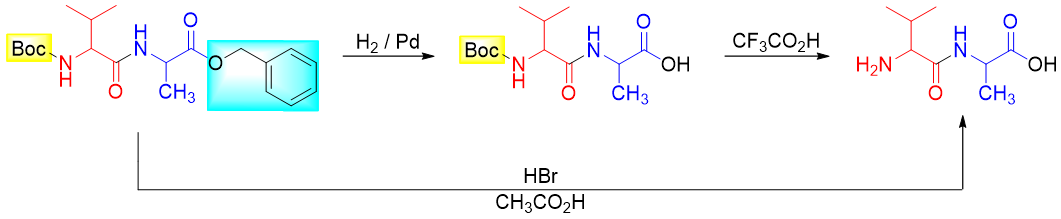
Finally, the protecting groups are removed either in one step or in sequence.
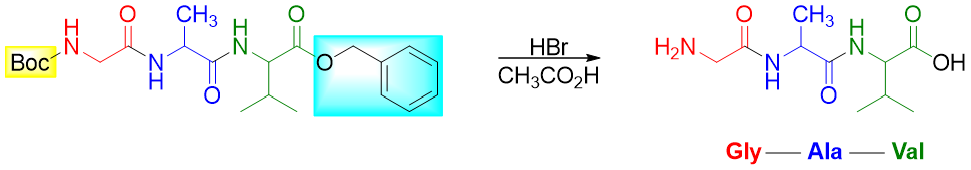
This strategy can be applied to prepare tripeptides and polypeptides. In tripeptide synthesis, once the protected dipeptide is formed, only one protecting group is removed and then reacted with another amino acid protected at unwanted functional group. For example, the Gly-Ala-Val tripeptide is formed in the following sequence.
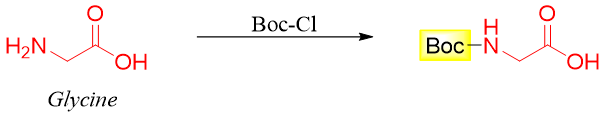
Step 1: Protect -NH2 group of Glycine
Step 2: Protect -COOH groups of Alanine and Valine
Step 3: Couple protected Glycine and Alanine
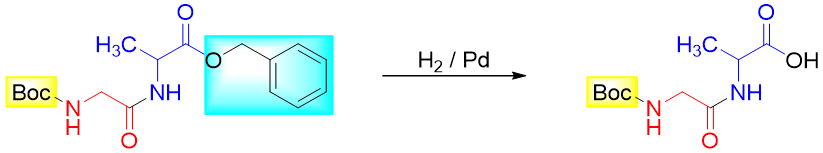
Step 5: Join the dipeptide (with unprotected -COOH group) with protected Valine
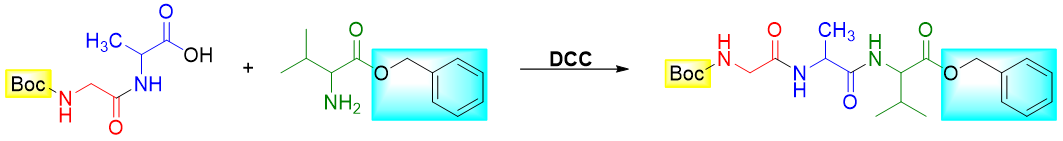
Step 6: Remove the protecting groups