Structure and Stereochemistry
Structure and Stereochemistry
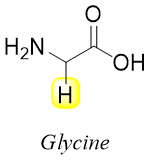
Amino acids contain amino groups and carboxylic groups. Since the amino group is bonded to alpha carbon they are usually referred as α-amino acids. The simplest amino acid is the amino acetic acid named glycine. In glycine the R substituent (side chain) is the hydrogen atom.
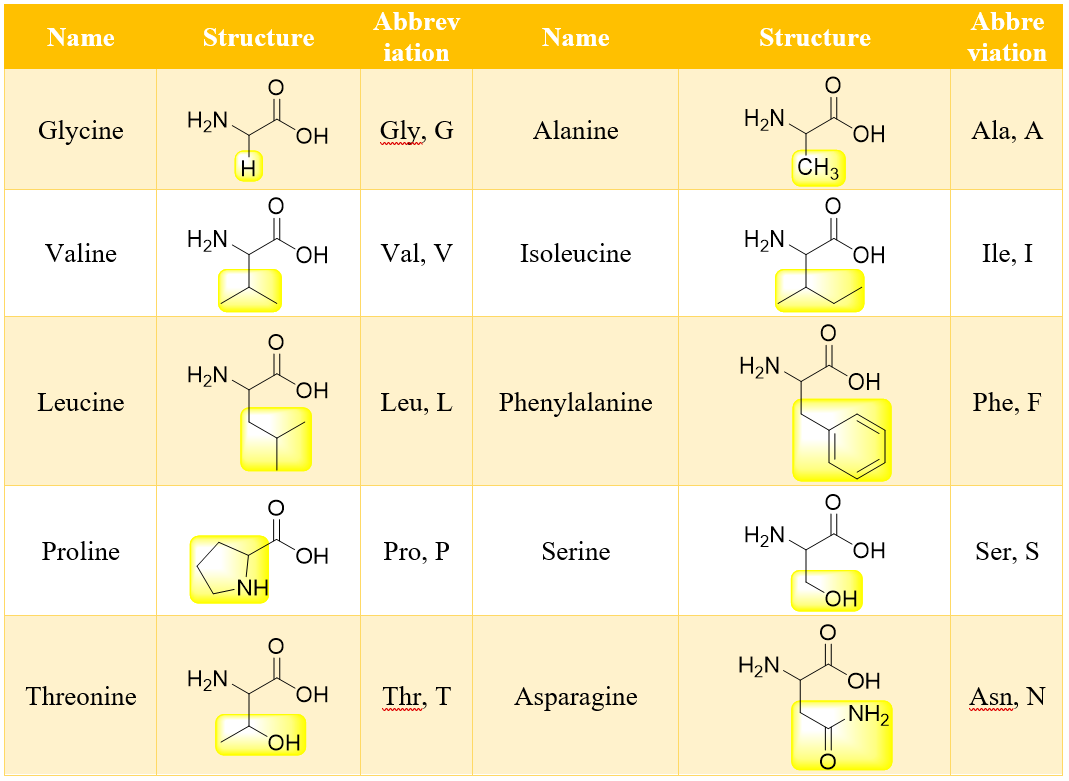
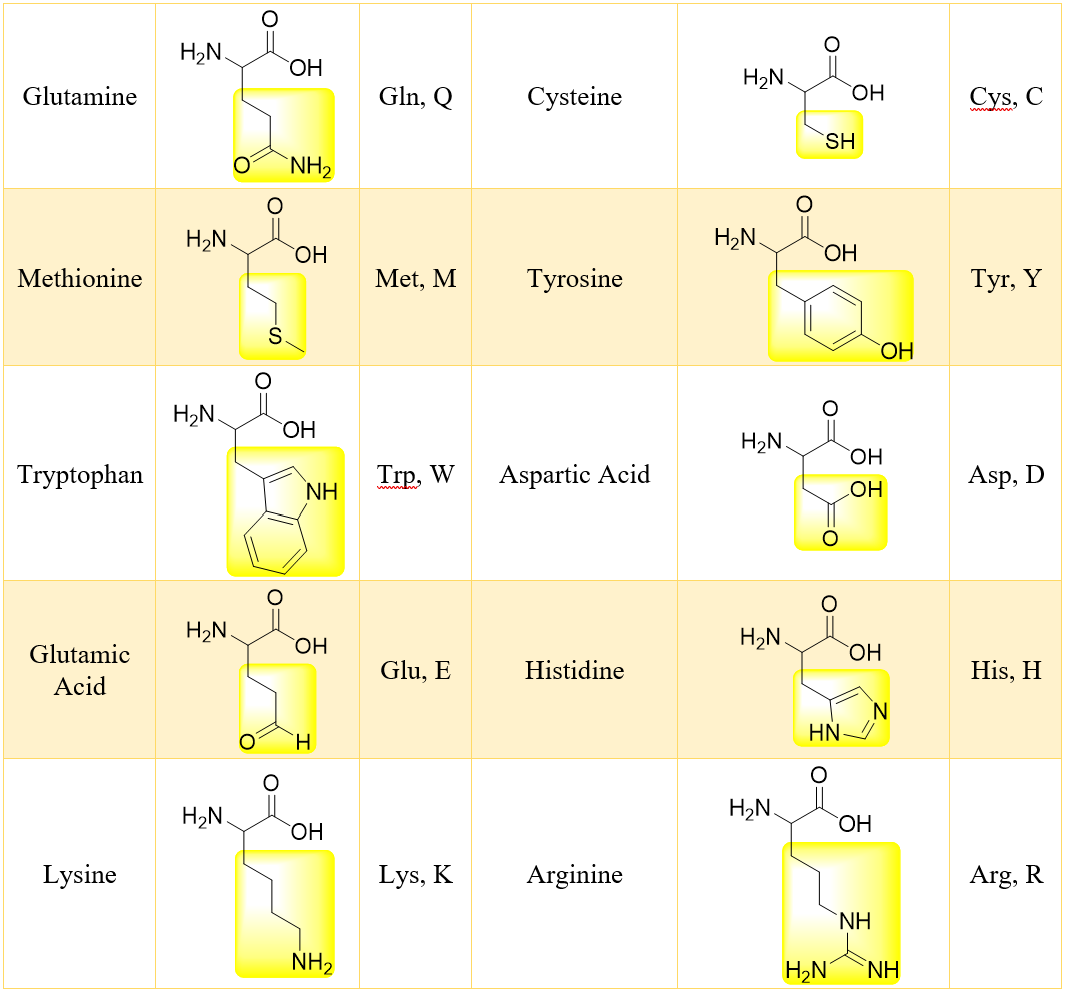
There are about twenty standard α-amino acids which differ only in the substituent R. Following table shows the structures of standard α-amino acids found in almost all proteins.
All amino acids except glycine are chiral in nature. Almost all naturally occurring amino acids have (S)-configuration at the alpha carbon. The stereochemistry of amino acids resembles the stereochemistry of L-(-)-glyceraldehyde therefore, they are also called L-amino acids.
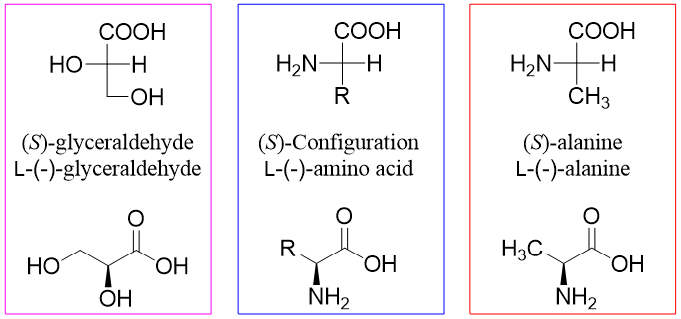
Occasionally D-amino acids are found in nature although, we assume that the amino acids under consideration are L-amino acids. The (+) and (-) notations are used for specifying the optical rotations of compounds containing chiral centers. These notations have no connection with R or S configurations and are determined experimentally.
Amino acids are further classified as essential and nonessential amino acids. Those amino acids which are synthesized by the animal body are the nonessential amino acids, and those which are not synthesized by the animal body and must be provided by diet are the essential amino acids. Following table shows the essential and nonessential amino acids.

The essential amino acids are provided to the animal body by taking proteins in daily diet. Those proteins which contain all essential amino acids are termed as complete proteins. Complete proteins are present in milk, eggs, fish, and meat. On the other hand, proteins which lack one or more essential amino acids are referred to as incomplete proteins. Proteins present in plants are mostly incomplete proteins.
In addition to twenty standard amino acids there are some other rare amino acids found in proteins. These rare amino acids include selenocysteine, pyrrolysine, 5-hydroxylysine, and 4-hydroxyproline.

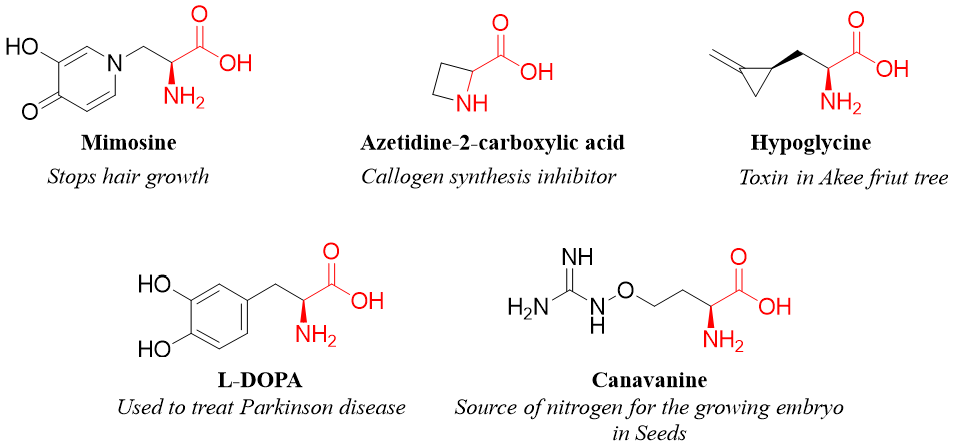
Furthermore, there are about 700 amino acids which are not involved in forming proteins. These amino acids are called nonprotein amino acids. For example,