Acid-Base Properties
Acid-Base Properties
Amino acids contain both amino groups and carboxyl groups. These functional groups make the amino acid either acidic or basic. The amino acid exists in the form of zwitterion.
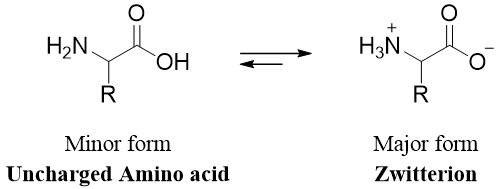
As amino acids exist mostly in zwitterion form therefore, the acidity of amino acids is due to the -NH3+ group and the basicity is due to the -COO- group. The zwitterion is also responsible for giving amino acids some remarkable physical properties. Amino acids have high melting points, are highly soluble in water, less soluble in non-polar solvents, and have larger dipole moments. Each amino acid has a prominent form which directly depends upon the pH of the solution. In acidic solutions (smaller pH) the -COO- (carboxylate) ion is protonated to form carboxylic acid and the overall charge on amino acid becomes positive.
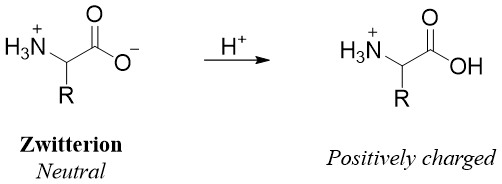
When the pH of the solution is increased, if there is any -COOH group present, it is deprotonated first followed by deprotonation of the -NH3+ ion into the -NH2 group making the overall molecule negatively charged.

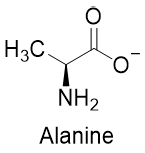
For example, the pKa value of the -COOH group of alanine is 2.34 and the pKa value of the -NH3+ group is 9.69.
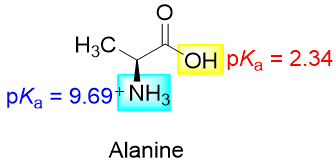
If the above alanine is added to an acidic solution with pH smaller than 2.34 then the alanine will exist in the same form as shown above. If the pH of the solution is greater than 2.34 and smaller than 9.69 then the -COOH will lose its proton (first dissociation constant) and alanine will exist in the following form.
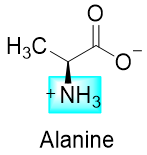
And if the pH of the solution is greater than 9.69 then the -NH3+ group will lose its proton (second dissociation constant) and alanine will exist in the following form.
Following table shows the pKa values of all standard amino acids.
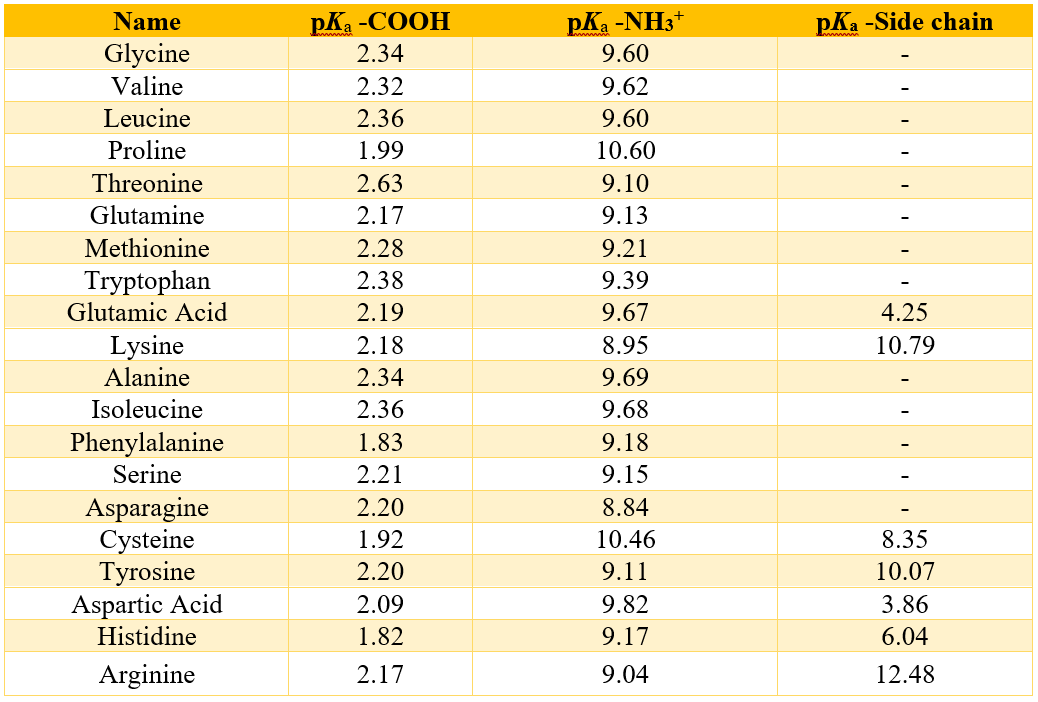
As shown in the above table, none of the standard amino acids can exist in a neutral uncharged form. For an amino acid to become neutral the -NH3+ group must lose a proton. In that case the -COOH group will lose protons because to deprotonate the -NH3+ group the pH must be greater than 7. Therefore, the pKa values -COOH groups of all amino acids are lower than the pKa values of -NH3+.
Following graph shows the titration curve for alanine. At pH below 2.34 alanine mostly exists in cationic form. With addition of base the pH of the system increases. At pH about 6.0 (isoelectric pH) the alanine entirely exists in the form of zwitterion. At pH above 9.69 the alanine exists in anionic form. This titration method of amino acids is used to separate desired forms of amino acids.
