Enzymes and Coenzymes
Enzymes and Coenzymes
Enzymes are proteins which catalyze different biological reactions. Like catalysts, enzymes are not involved in changing the equilibrium of the reaction; instead, they participate in decreasing the activation energy of the biological reaction and increasing the rate of reaction.

Above energy diagram shows that the reaction without enzymes has higher activation energy and in the presence of enzymes the activation energy decreases. In the presence of enzymes, the rate of reactions commonly increases by a million fold. For example, the enzyme glucosidase increases the rate of hydrolysis of polysaccharide by the factor of more than 1017. Without this enzyme the reaction would take millions of years to complete.
Unlike catalysts, enzymes are extremely specific, and each enzyme catalyzes only a particular reaction of a specific substrate. Following table shows some enzymes which specifically convert given substrates to products.
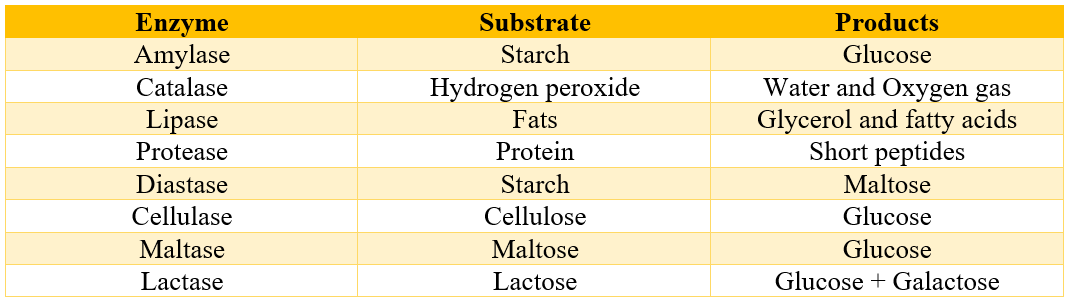
Some enzymes are found to work on a range of substrates. For example, carboxypeptidase can assist in the removal of any amino acid residue present at the C-terminus and Papain that can hydrolyze any peptide bond.
How does Enzyme work?
Enzymes engage in reducing the activation energy of the reaction. Every enzyme has an active site which contains specific amino acids. The amino acid residues of the active site interact with substrate and form an intermediate. This intermediate complex has a lower energy. The second reactant upon reaction with this intermediate forms the final product and the enzyme is reformed. Following figure represents the Lock and Key hypothesis of enzyme working. In this hypothesis the substrates fit into the active site of the enzyme and form an enzyme-substrate complex (intermediate). In this model the enzyme acts as the lock and the substrate acts as the key. Only certain substrates can fit into the active site with specific shape.
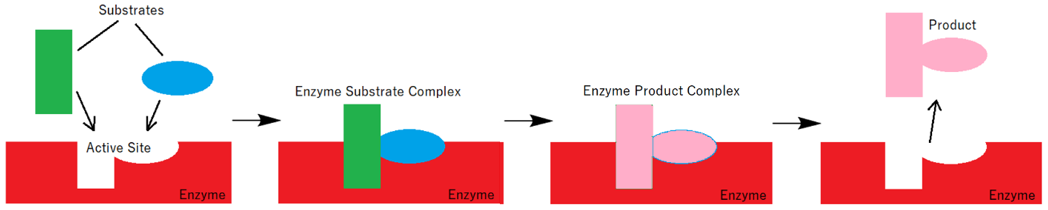
The Lock and Key model
Another model which explains the working of an enzyme is the Induced Fit model. According to this model the shape of the active site changes in order to fit the incoming substrate(s). Hence the change in the shape of the active site is induced by the incoming substrate. This model shows that the protein structure is flexible and can make changes in its conformations.
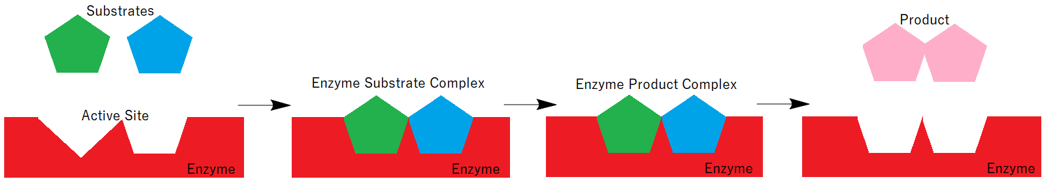
The Induced fit model
The number of substrate molecules converted into corresponding product(s) by an enzyme in one minute is called the turnover number. A typical turnover number for an enzyme is about 1000 but some enzymes like carbonic anhydrase can reach the turnover number value of 600,000.
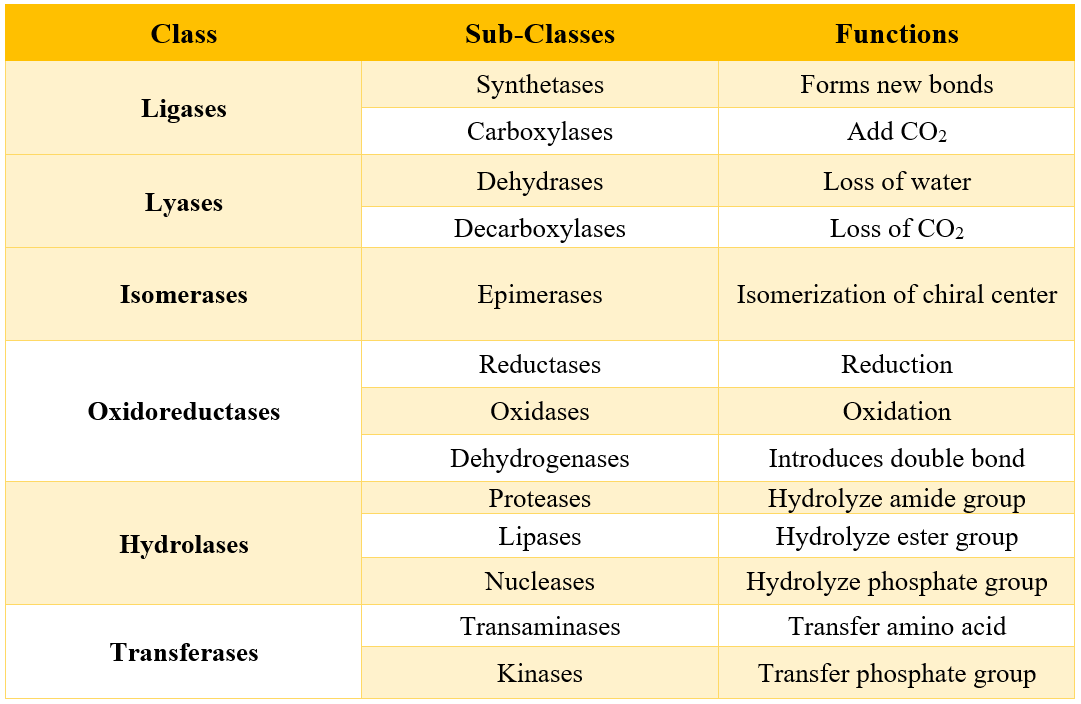
Enzymes are categorized into six classes depending upon the type of reactions they catalyze. These classes are summarized in the following table.
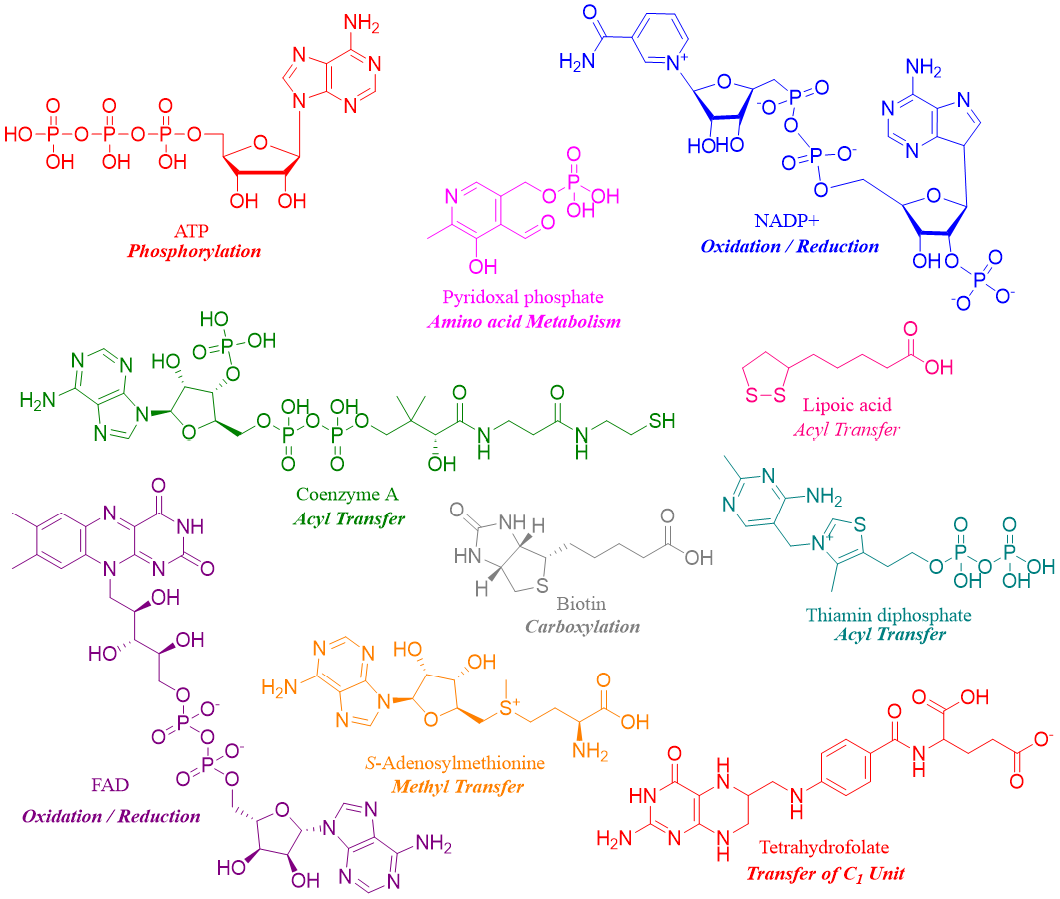
Along with the protein part most of the enzymes also contain non-protein part called cofactor. The cofactor can be a small organic molecule called coenzyme or it can be an inorganic ion like Zn2+, Fe3+, Fe2+, Cu2+, K+ or Mg2+ etc. Coenzyme is not a catalyst itself, but it binds to the active site of the enzyme to assist the catalytic reaction. More specifically, coenzymes function as intermediate electron carriers and transfer electrons throughout the reaction. Structures and functions of some coenzymes are shown below.