Transesterification
Transesterification
In transesterification reactions of esters, one alkoxy group (RO-) is substituted by another different alkoxy group. These reactions are either acid catalyzed or base catalyzed.
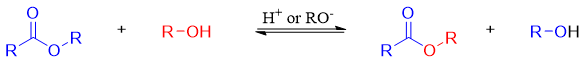
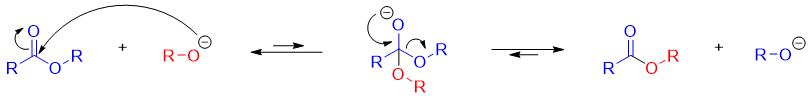
The mechanism of transesterification is identical to the mechanism for ester hydrolysis except that the nucleophile in ester hydrolysis is H2O while in transesterification it is ROH.
Following the mechanism of acid catalyzed transesterification reaction.
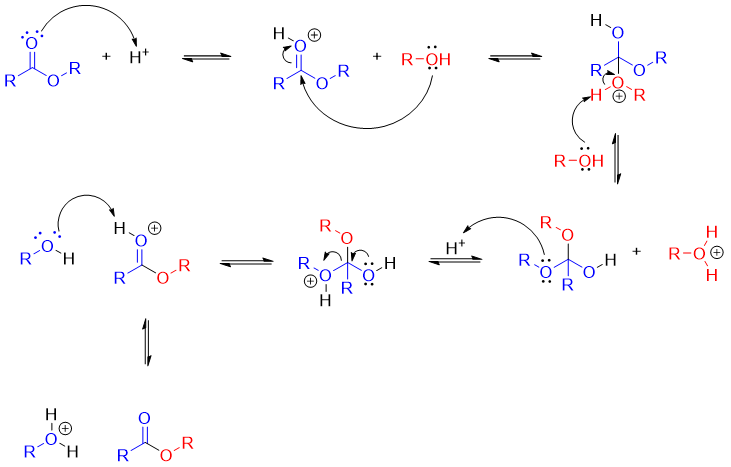
As shown in mechanism the tetrahedral intermediate has two different alkoxy groups as a leaving group and both have almost the same basicities. For the purpose, the alcohol is added in excess to produce desired product in good yields. For example
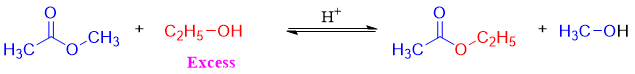
The transesterification reaction is also base catalyzed. The mechanism for base catalyzed transesterification is shown below.
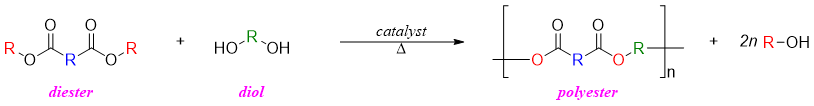
The process of transesterification has many applications. This process is used to synthesize polyesters on large scale. In polymerization diesters are reacted with diol to form polymers. For example
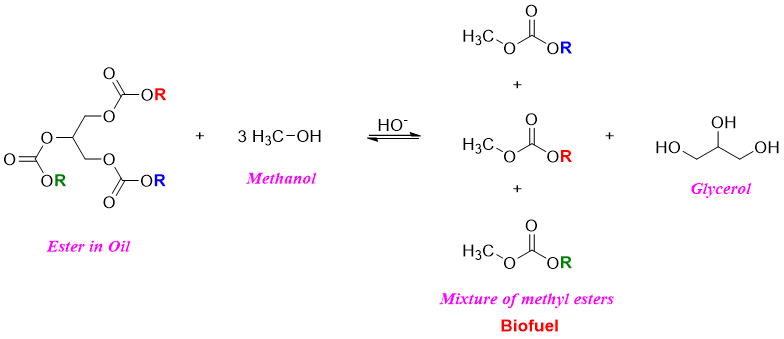
The process of transesterification is also used to produce bio diesel from both unused vegetable oils and waste cooking oil. The triglycerides are treated with methanol in the presence of base catalyst to produce mixture of alkyl esters (biofuel) and glycerol.
Above mentioned procedure can also be employed to recycle different polyester polymers.
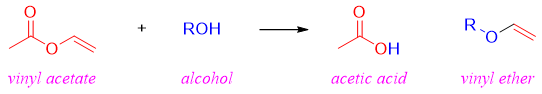
Transesterification reaction is also employed to synthesize derivatives of vinyl ethers. Vinyl ethers are difficult to synthesize by other methods. Vinyl acetate which is cheaply available is reacted with different alcohols to produce derivatives of vinyl ethers.