Alpha Reactions
Alpha Reactions
The alpha substitution reactions are those reactions in which a substitute is placed at a carbon next to the carbonyl group. This position is called α-position, the carbon is called α-carbon and the hydrogen attached to α-carbon is called α-hydrogen. In alpha substitution reactions the α-hydrogen(s) are typically replaced by an electrophile.
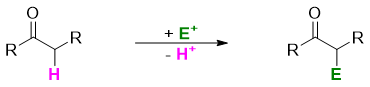
The α-hydrogen is acidic in nature. This acidity is due to the stability of conjugate base (enolate). The enolate is stabilized by resonance originated by the delocalization of negative charge over α-carbon and carbonyl group.
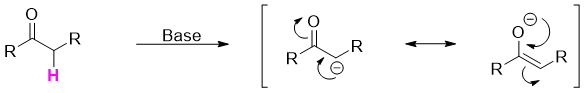
The negatively charged enolate acts as nucleophile and attacks on electrophile to replace the α-hydrogens.
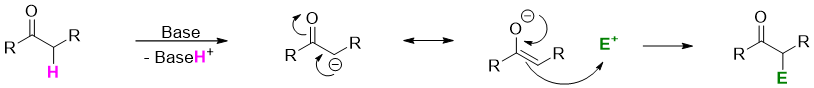
Some common α-substitution reactions are discussed below.
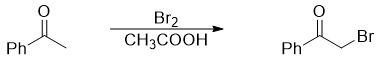
Alpha Halogenation of Aldehydes and Ketones
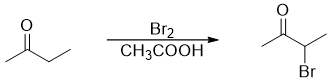
Aldehydes and ketones containing α -hydrogens when treated with Cl2, Br2, or I2 in acidic medium undergoes alpha halogenation reaction. For example
Mechanism:
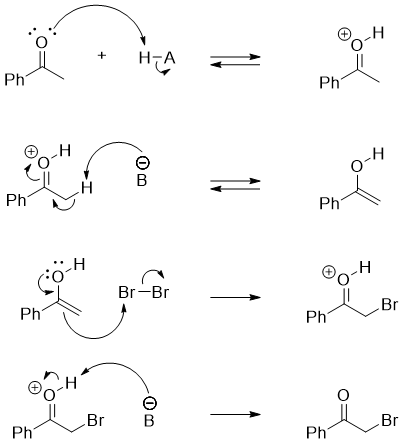
If the ketone is unsymmetrical then the alpha substitution will take place at the alpha carbon containing a smaller number of α-hydrogens as the highly substituted enol is favored over the less substituted enol. For example
Haloform Reaction
Most ketones undergo halogenation reaction in the presence of base until all alpha hydrogens are replaced by halogen atoms.

The three halogen atoms on alpha carbon makes the trihalomethyl group a good leaving group in nucleophilic acyl substitution reactions. When treated with hydroxide ion, the trihalomethyl ketone forms a tetrahedral intermediate which expels trihalomethyl anion and forms carboxylic acid. The carboxylic acid is deprotonated by the trihalomethyl anion to for haloform and carboxylate ion. This overall reaction is called as Haloform reaction.
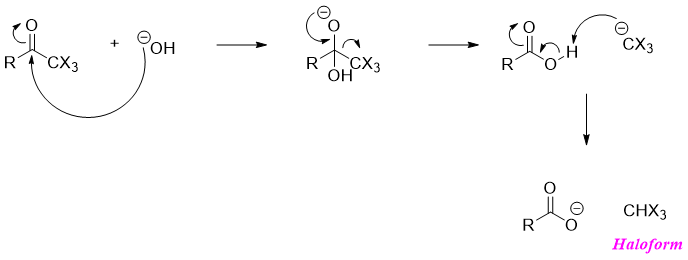
When the halogen is iodine then the haloform produced is Iodoform. Iodoform separates as a sold precipitate and is yellow in color. This iodoform test is used to identify methyl ketones.
Alkylation of Enolate Ion
Enolate ion when treated with alkyl halide or tosylate results in alkylation reaction. In this way two small molecule are connected via new C-C bond and forming a large molecule.
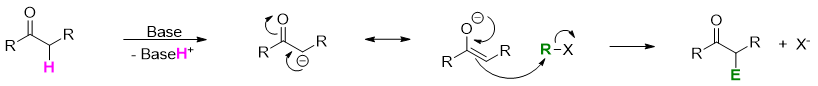
The reaction between enolate and alkyl halide follows SN2 mechanism. The R group is either primary or methyl (preferably allylic or benzylic) and the X group is the leaving group (Tosylate, Cl-, I-, or Br-).
The bases like alkoxide or hydroxide can not be used for the formation of enoles as these bases can directly react with the alkyl halides and produce unwanted byproducts. For this purpose, Lithium diisopropylamide (LDA) is used to avoid unwanted side reactions. LDA is a bulky base and a poor nucleophile and does not react with alkyl halides or tosylates.
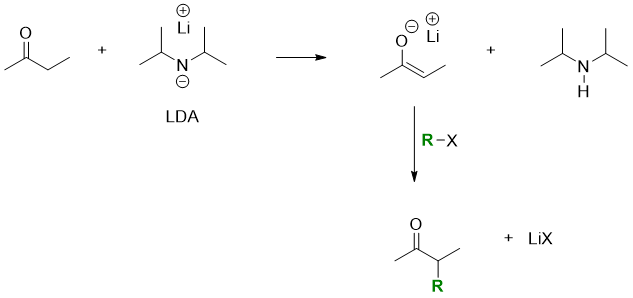
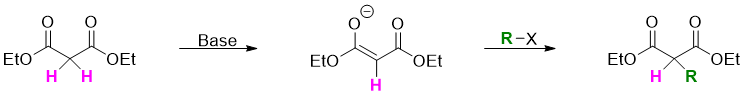
1,3-diesters like diethyl propanedioate are relatively more acidic (pKa = 13) as the alpha hydrogens are flanked by two carbonyl groups. It is easily converted to enolate when reacted with base. The enolate reacts with alkyl halides to produce alpha substituted malonic ester.
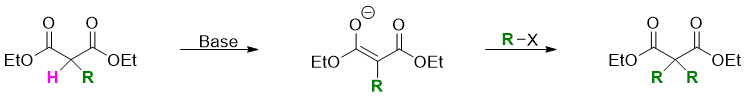
The alkylated malonic ester still has another acidic alpha hydrogen thus, it undergoes another alkylation reaction to produce dialkylated malonic ester.
When monoalkylated or dialkylated malonic ester is heated with HCl, hydrolysis takes place followed by decarboxylation reaction. This loss of CO2 results in the formation of mono carboxylic acid.

The mechanism of evolution of CO2 is shown below.
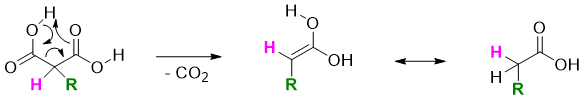
The dicarboxylic reaction is unique to compounds containing carbonyl group at beta position to carboxylic acid.
