Claisen Condensation
Claisen Condensation
Another important class of carbon centered nucleophiles are the enolates. Enolates are type of alkenes. Enolates are formed when a carbonyl compound having alpha proton is treated with a base. The base abstracts the alpha hydrogen atom as it is acidic in nature.
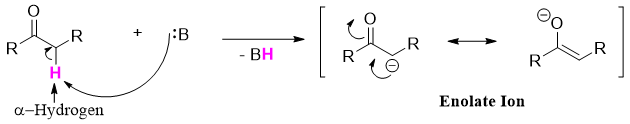
The α-hydrogens of esters are weakly acidic and can be deprotonated to produce enolate ions. The α-hydrogens of aldehydes and ketones are more acidic than esters because the oxygen atom of alkoxy group stabilizes the enolate ion through resonance.
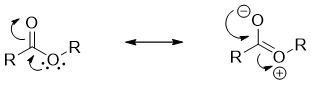
The pKa value of α proton of an ester is about 24 and pKa of aldehydes and ketones is about 20. The smaller the pKa values the more the acidic a proton and vice versa.
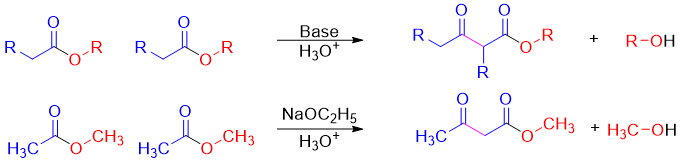
Esters can act as an enolate (nucleophile) or as an electrophile in enolate reactions. These reactions are called Claisen Condensation reactions. In these reactions the new carbon-carbon bond can form between two identical esters (self-Claisen condensation) or between two different esters or between an ester and other carbonyl compound (crossed-Claisen condensation). Following reaction is an example of self-Claisen condensation.
Mechanism:
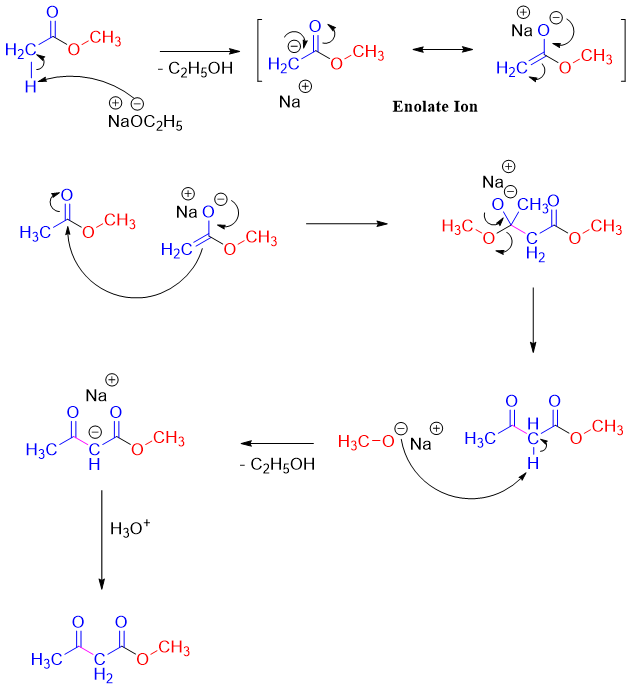
The β-keto ester formed in Claisen condensation reactions are more acidic than aldehydes and ketones with pKa value of about 11. This increase in acidity is due to the stabilization of the negative charge over both carbonyl groups.
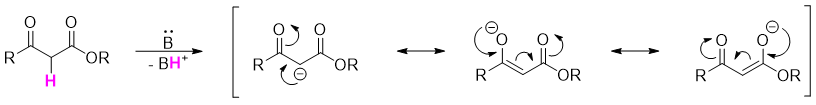
The Claisen condensation is a reversible reaction and favors the reactants as the esters are more stable than the β-keto esters. Obeying Le Châtelier’s principle, the reaction can be shifted in forward direction by deprotonating the β-keto esters. For this purpose, the base is added in equivalent amounts instead of catalytic amounts. Once the reaction is completed acidic workup is performed to reprotonate the β-keto esters.
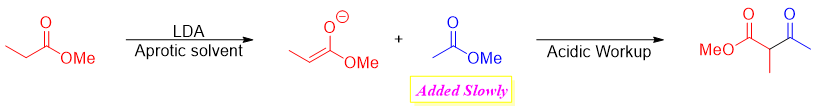
In cross-Claisen condensation two different carbonyl compounds are reacted. The carbonyl compound having alpha hydrogen is converted into an enolate. The second carbonyl compound is often lacks alpha hydrogen to avoid unwanted byproducts. For example
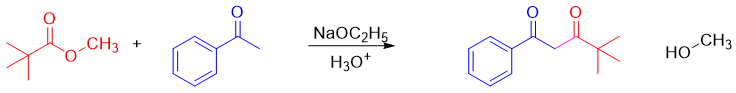
Mechanism:
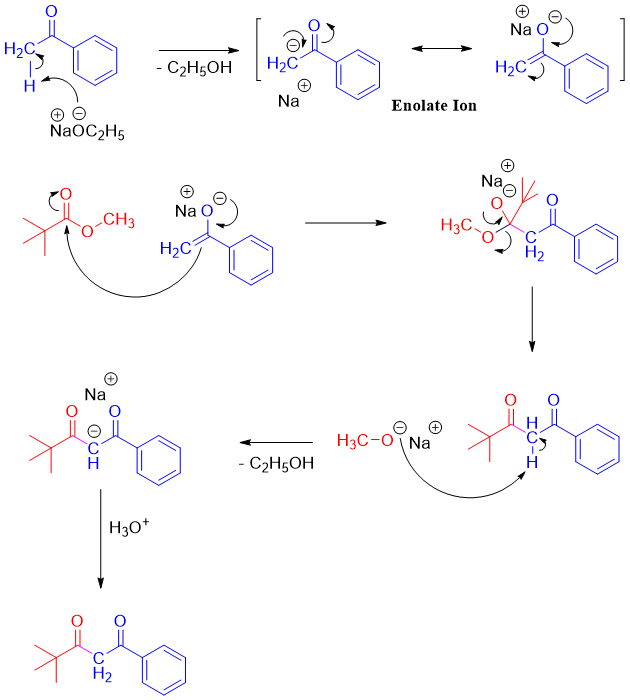
If both reactants have α-hydrogens, then one product can primarily be formed reacting one reactant with Lithium diisopropylamide (LDA) to produce desire enolate. The other carbonyl reactant is then added slowly to make sure it reacts completely with the enolate present in the reaction mixture and minimize the chances of its conversion to enolate.