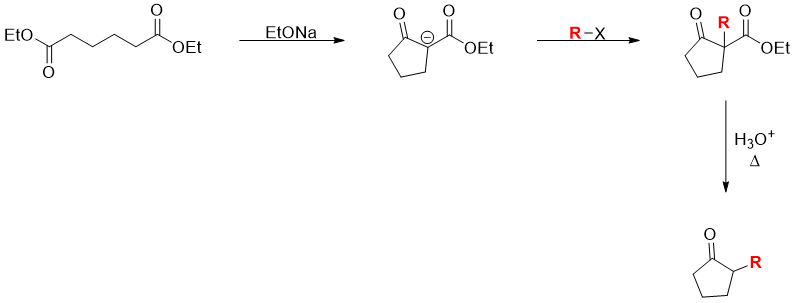
Dieckmann Condensation
Dieckmann Condensation
Diester can undergo intramolecular Claisen condensation when treated with base. This reaction is called as Dieckmann cyclization. These reactions work on 1,7 diesters and 1,6 diesters which produce six-membered cyclic β-keto esters and five-membered cyclic β-keto esters respectively.
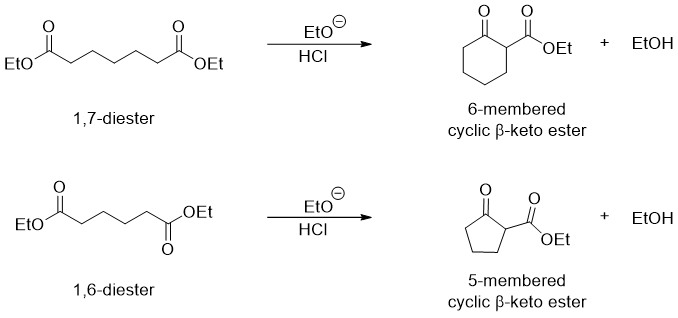
The Dieckmann reaction can be driven to completion by adding equivalent amounts of base instead adding bases in catalytic amounts. The mechanism of Dieckmann cyclization is the same as Claisen Condensation reactions.
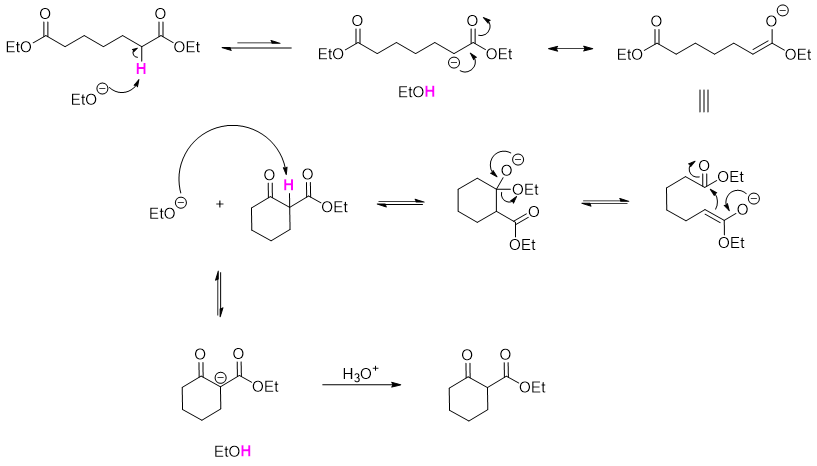
The mechanism of conversion of 1,6-diester to 5-membered cyclic β-keto ester is given below.
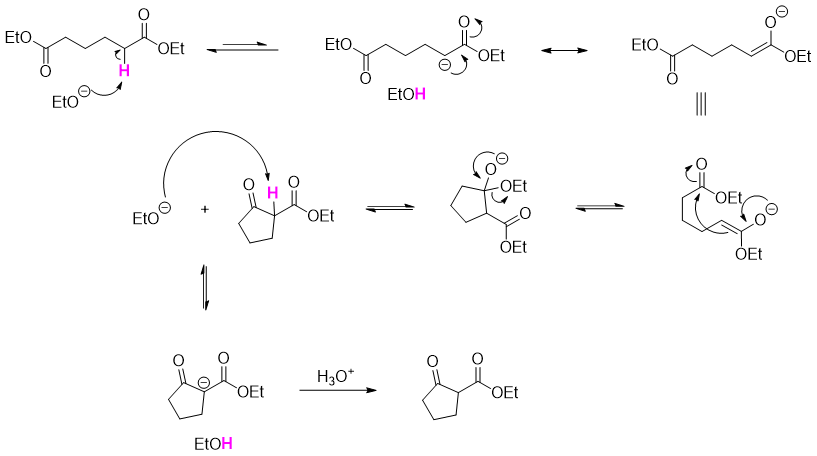
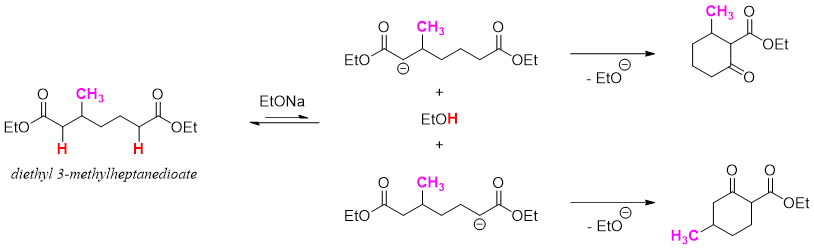
Dieckmann cyclization of unsymmetrical substituted diesters produces a mixture of products. In following reaction diethyl 3-methylheptanedioate upon Dieckmann cyclization produces two possible products.
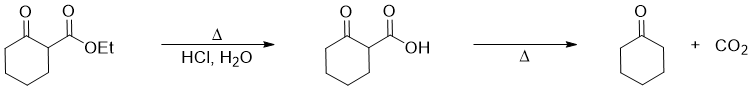
The ester group in cyclic β-keto esters can be hydrolyzed to carboxylic acid group. The carboxylic group can further be removed by heating and producing ketone as a product. This reaction is called as decarboxylation reaction.
In organic synthesis Dieckmann cyclization along with other reactions are often utilized to produce desire products. For example, α-alkylated ketones are produced by performing i) Dieckmann Cyclization followed by ii) α-Alkylation and iii) decarboxylation reaction.