Reduction of Esters
Reduction of Esters
Esters upon reaction with lithium aluminum hydride (LiAlH4) undergoes hydride reduction and forms two alcohols. The hydride reduction of esters cannot be achieved by treating it with sodium borohydride (NaBH4). NaBH4 is not reactive enough to reduce ester to corresponding alcohols. The general reaction for the reduction of ester with LiAlH4 is given below.
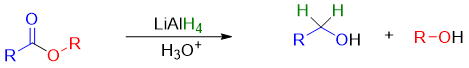
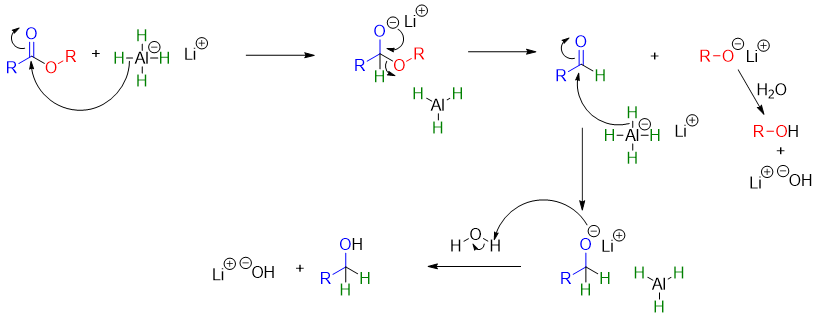
Mechanism:
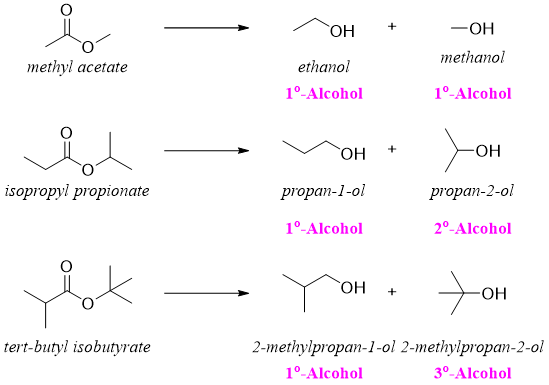
In this rection two alcohols are formed. The alcohol derived from carbonyl part is the main product and this alcohol is primary alcohol while, the second alcohol derived from alkoxide part of ester can be primary, secondary, or tertiary. For example.
As observed in above mechanism the conversion of ester to primary alcohol went through an aldehyde. The aldehyde formed in situ was further reduced by LiAlH4.
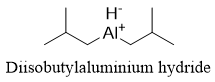
It is possible to stop the reaction at aldehyde by using different hydride reagent. The modified hydride reagent used for the conversion of ester into aldehydes is called Diisobutylaluminium hydride (DIBAL-H). DIBAL-H is a weaker reducing agent compared to LiAlH4. The structure of DIBAL-H is shown below.
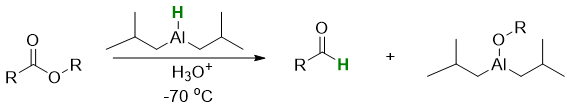
DIBAL-H reduces esters to aldehydes.
Mechanism:
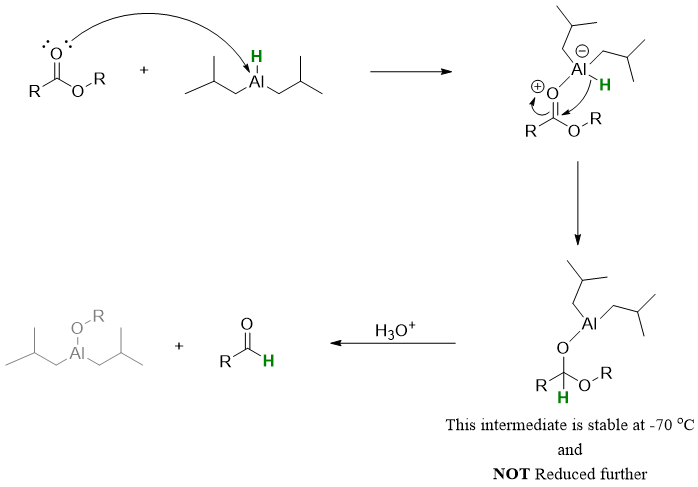
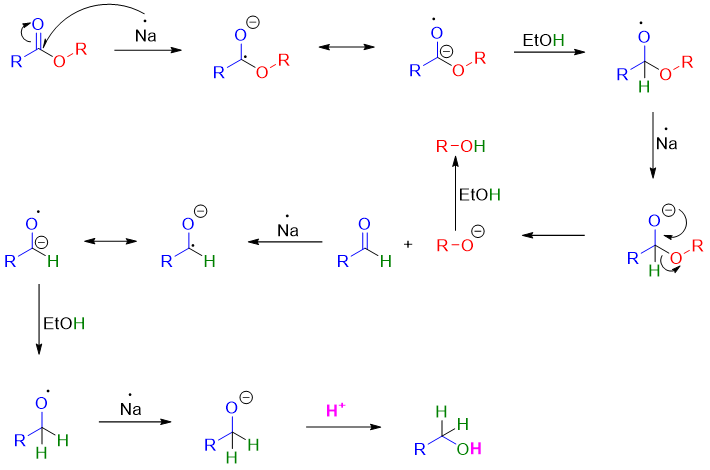
Esters can also be reduced to alcohols by treating esters with absolute ethanol in the presence of sodium metal. This reaction is also called Bouveault-Blanc Reduction. This method is used an alternate of LiAlH4 in reduction of esters at large scale productions

Mechanism: